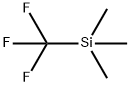
(Trifluoromethyl)trimethylsilane: Transformative Role in Modern Organic Synthesis and its Preparation Method
Oct 22,2024
General Description
The trifluoromethyl group (CF3-) has a privileged role in pharmaceuticals and agrochemicals because its incorporation into drug candidates could enhance chemical and metabolic stability, improve lipophilicity and bioavailability, and increase protein binding affinity. Additionally, trifluoromethylated organic compounds are widely applied in materials such as liquid crystals. While there are no naturally occurring CF3- containing compounds, it is not surprising that numerous efforts have been devoted to the development of new methodologies for the preparation of these compounds. Direct trifluoromethylations, especially transition-metal-mediated or catalyzed trifluoromethylation reactions, are the most promising and efficient route to access a wide range of CF3- containing molecules. Methods for the incorporation of the trifluoromethyl group into organic molecules may be considered as nucleophilic, electrophilic, or free radical processes. This article will introduce the use of (Trifluoromethyl)trimethylsilane, and the preparation procedure.

Figure 1. (Trifluoromethyl)trimethylsilane
Transformative Role in Modern Organic Synthesis
In this paper1, Lingling Chu and Feng-Ling Qing describe their studies of oxidative trifluoromethylation reactions of various nucleophiles with (Trifluoromethyl)trimethylsilane (CF3SiMe3) in the presence of oxidants. They have focused most of our efforts on constructing carbon−CF3 bonds via direct trifluoromethylation of various C−H bonds, and have demonstrated copper-mediated or -catalyzed or metal-free oxidative C−H trifluoromethylation of terminal alkynes, tertiary amines, arenes and heteroarenes, and terminal alkenes. Besides various C−H bonds, aryl boronic acids proved to be viable nucleophilic coupling partners for copper-mediated or -catalyzed cross-coupling reactions with CF3SiMe 3.
To further expand the reaction scope, authors also applied H-phosphonates to the oxidative trifluoromethylation system to construct P−CF3 bonds. Most recently, they developed silver-catalyzed hydrotrifluoromethylation of unactivated olefins. These studies explore boronic acids, C−H bonds, and P−H bonds as novel nucleophiles in transition-metal-mediated or -catalyzed cross-coupling reactions with CF3SiMe3, opening new viewpoints for future trifluoromethylation reactions.
Furthermore, authors also achieved the oxidative trifluoromethylthiolation reactions of aryl boronic acids and terminal alkynes to construct carbon−SCF 3 bonds by using CF3SiMe3 and elemental sulfur as the nucleophilic trifluoromethylthiolating reagent. These oxidative trifluoromethylation and trifluoromethylthiolation reactions tolerate a wide range of functional groups, affording a diverse array of CF3- and CF3S-containing compounds with high efficiencies, and provide elegant and complementary alternatives to classical trifluoromethylation and trifluoromethylthiolation reactions. Because of the importance of the CF3 and SCF3 moieties in pharmaceuticals and agrochemicals, these reactions would have potential applications in the life science fields.
Preparation Method
Patent CN106117258A provided that a kind of technique is simple, low cost, yield are high, safety The preparation method of (Trifluoromethyl)trimethylsilane of environmental protection.
It solves the scheme of above-mentioned technical problem employing: the preparation side of a kind of (Trifluoromethyl)trimethylsilane Method, comprises the following steps:
A, under argon shield, metallic potassium, styrene and hexamethyldisiloxane carry out reacting to metallic potassium in toluene Being completely dissolved, the mol ratio of described metallic potassium, styrene and hexamethyldisiloxane is 1:1-2:1-2, and described reaction temperature is 40-80 DEG C, after reaction terminates, it is cooled to room temperature, obtains reactant liquor;
B, at-45~-85 DEG C, adds trim,ethylchlorosilane in the reactant liquor that step (a) obtains, and is passed through fluoroform Reacting, described trim,ethylchlorosilane is 1:1-1.5 with the mol ratio of the metallic potassium described in step (a), when being passed through fluoroform Amount and trim,ethylchlorosilane mol ratio when being 1:1-1.5, stop being passed through fluoroform, be incubated 1-3h, be warming up to room temperature, by obtain Reactant washing, stratification obtain organic layer, and organic layer rectification is obtained product (Trifluoromethyl)trimethylsilane.
References:
[1] LINGLING CHU F L Q. Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)trimethylsilane as a Nucleophilic CF3 Source[J]. Accounts of Chemical Research, 2014, 47 5: 1455-1642. DOI:10.1021/ar4003202.
- Related articles
- Related Qustion
- Trifluoromethyl(trimethylsilane): A Versatile Reagent in Organic Synthesis Jun 27, 2023
Trifluoromethyl(trimethylsilane) is a useful chemical reagent that contains a trifluoromethyl group and a trimethylsilyl group. It is commonly used as a source of the trifluoromethyl group.
- Instroduction of (Trifluoromethyl)trimethylsilane Nov 9, 2021
(Trifluoromethyl)trimethylsilane belong to organic fluorine chemistry has made rapid progress in recent decades, and the research and development of fluorine-containing compounds have had a great impa
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringN,O-Dimethylhydroxylamine hydrochloride enhances dental materials, acts as antimicrobial in periodontal care, and improves biomaterial integration, with safety precautions essential.....
Jul 31,2024API(Trifluoromethyl)trimethylsilane
81290-20-2You may like
(Trifluoromethyl)trimethylsilane manufacturers
- (Trifluoromethyl)trimethylsilane
-
- $8.00 / 1KG
- 2025-09-25
- CAS:81290-20-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: g-kg-tons, free sample is available
- (Trifluoromethyl)trimethylsilane
-

- $0.00 / 25KG
- 2025-08-28
- CAS:81290-20-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month
- (Trifluoromethyl)trimethylsilane
-

- $0.00 / 1KG
- 2025-06-27
- CAS:81290-20-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg