1,1,1,2-Tetrafluoroethane: Properties, Production process and Uses
Mar 20,2024
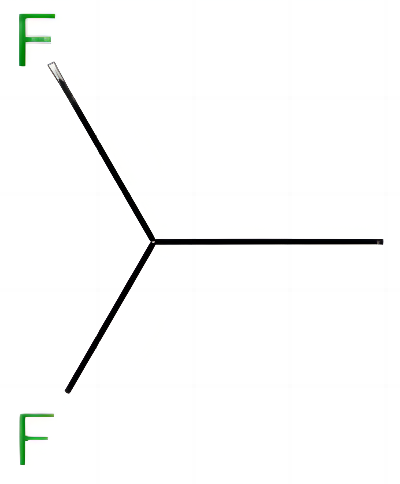
1,1,1,2-Tetrafluoroethane, also known as norfluran, R134a, 1,2,2,2-tetrafluoroethane and HFC-134a, is a derivative of trichloroethylene or tetrachloroethylene. Molecular formula: C2H2F4, molecular weight: 102.0309. The chemical structure is:
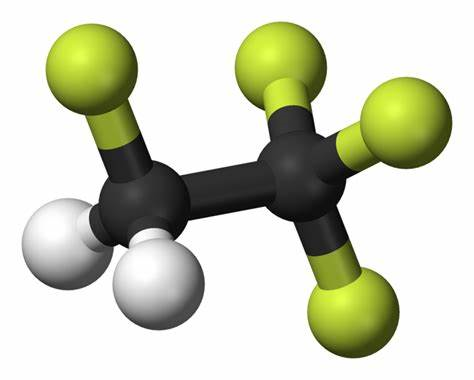
Properties of tetrafluoroethane
Tetrafluoroethane (HFC-134a) is a colorless and nonflammable chemical substance with a boiling point of −26.6°C, a saturated vapor pressure of 661.9 kPa (25°C) and an enthalpy of vaporization of 216 kJ kg–1 (1 atm). It is insoluble in water but soluble in ether. The lack of chlorine in the molecule reduces the ozone depletion activity to practically zero. The thermodynamic performance is very similar to CFC-12 (boiling point: −29.8°C), and it is also comparable in safety to CFC-12. Therefore, it is regarded as the best alternative to CFC-12. Although HFC-134a has a certain greenhouse effect (HGWP = 0.28), which does not affect it as the preferred ODS (Ozone Depleting Substance) alternative. Since the mid-1990s, HFC-134a has been widely used in the industrial and commercial refrigeration, automotive air conditioning, central air conditioning, household refrigerators, plastic foam, pharmaceuticals, cosmetic aerosols and medical aerosol propellants. In recent years, tetrafluoroethane is also used as an extraction solvent.
Production process of tetrafluoroethane
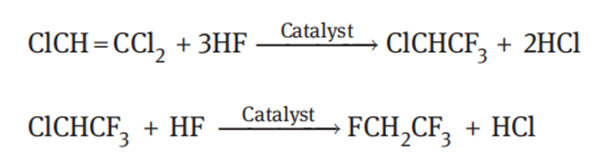
Trichloroethylene route
In the presence of a catalyst, fluorination of trichloroethylene with hydrogen fluoride generates 1,1,1-trifluoro-2-chloroethane (HCFC-133a); then, at higher temperature, fluorination of 1,1,1-trifluoro-2-chloroethane gives 1,1,1,2-tetrafluoroethane (HFC-134a):
Tetrachloroethylene route
Tetrafluoroethane is produced from tetrachloroethylene and hydrogen fluoride. The reaction equations are as follows:
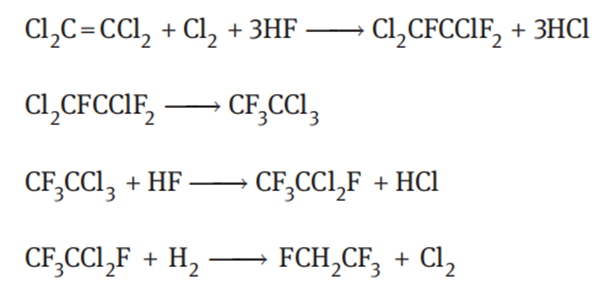
Using chromium plus gold as the catalyst, the conversion of tetrachloroethylene per pass was 50% and the selectivity to tetrafluoroethane was 80%. DuPont developed three generations of synthetic technologies. Compared to the first-generation technology, the reactors and catalysts were improved and the production capacity of the reactor with the same volume was doubled in the second-generation technology. The third-generation technology does not require a catalyst, but requires strict temperature control.
This process produces both HFC-134a and HCFC-124. If HFC-134a is the only prod -
uct required, all of the HCFC-124 are recycled. The moisture and nitrogen contents in the hydrogen should be less than 10 × 10−6 and 1,000 × 10−6, respectively. The hydrogen
is purified by pressure swing adsorption.
Uses of HFC-134a
HFC-134a is the most widely used medium- and low-temperature environmentally friendly refrigerant. Due to the good comprehensive performance of HFC-134a, it is a very effective and safe alternative to CFC-12 and mainly used as a refrigerant in refrigerators, freezers, air conditioners, dehumidifiers, cold storage, ice water machines, ice cream machines, refrigeration condensing units and so on. It can also be applied as aerosol propellant, polymer (plastic) physical foaming agent and magnesium alloy shielding gas.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringAccording to different raw materials, the processes for manufacture of 1,1-difluoroethane include two routes: fluorination of acetylene and fluorination of vinyl chloride.....
Mar 20,2024API1,1,1,2-Tetrafluoroethane
811-97-2You may like
- Rolapitant Synthesis
Dec 22, 2025
- Synthesis of 2-(2-Chlorophenyl)cyclohexanone
Dec 22, 2025
- Preparation methods and application of 2-(2-Ethoxyethoxy)ethyl acrylate
Dec 22, 2025