1-Hydroxycyclohexyl Phenyl Ketone: Role in Organic Synthesis and its Detection Method
Sep 10,2024
General Description
1-Hydroxycyclohexyl phenyl ketone is a vital compound in organic synthesis, particularly in the oxidation of primary alcohols and aldehydes to carboxylic acids. Its unique ability to mediate hydride transfer reactions under strongly basic conditions allows for efficient conversions while preserving various sensitive functional groups. This compound exhibits remarkable chemoselectivity, selectively oxidizing primary alcohols without affecting secondary ones, making it invaluable in targeted synthesis. Furthermore, 1-hydroxycyclohexyl phenyl ketone's detection in aqueous solutions using gas chromatography-mass spectrometry highlights its potential cytotoxicity, necessitating careful monitoring due to adverse health risks associated with its accumulation in clinical settings.

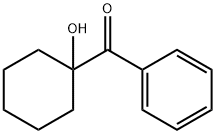
Figure 1. 1-Hydroxycyclohexyl phenyl ketone
Role in Organic Synthesis
1-Hydroxycyclohexyl phenyl ketone plays a pivotal role in organic synthesis, specifically in the oxidation processes of primary alcohols and aldehydes into their corresponding carboxylic acids. The oxidation reaction facilitated by 1-hydroxycyclohexyl phenyl ketone represents an important pathway in synthetic organic chemistry, addressing vital transformations that are fundamental in various applications, including pharmaceuticals and fine chemicals. The unique property of 1-hydroxycyclohexyl phenyl ketone to mediate hydride transfer reactions opens new avenues for the efficient conversion of alcohols into acids, which are crucial intermediates in numerous chemical syntheses. 1
Mechanism and Conditions of Reaction
Under strongly basic conditions, such as those provided by sodium tert-butoxide, the application of 1-hydroxycyclohexyl phenyl ketone allows for oxidation reactions to occur smoothly at room temperature. This environment enhances the efficiency of 1-hydroxycyclohexyl phenyl ketone, ensuring that a range of functional groups remains intact throughout the reaction. The tolerance of 1-hydroxycyclohexyl phenyl ketone for sensitive moieties such as tert-butanesulfinamides, amines, and sulfides is particularly noteworthy. Such flexibility in functional group acceptance enables chemists to conduct more complex synthesis without the risk of side reactions that could compromise yield or purity. 1
Chemoselectivity and Practical Applications
One of the standout features of the use of 1-hydroxycyclohexyl phenyl ketone is its capacity for chemoselective oxidation. In many synthetic scenarios, the simultaneous presence of primary and secondary alcohols poses a challenge; however, 1-hydroxycyclohexyl phenyl ketone can selectively oxidize primary alcohols and aldehydes to carboxylic acids without affecting the secondary alcohols. This characteristic is invaluable for targeted synthesis, where the goal often includes obtaining specific products from a mixture of reactants. The application of 1-hydroxycyclohexyl phenyl ketone not only enhances reaction productivity but also contributes significantly to the development of innovative synthetic methodologies in various fields of chemical research. 1
Detection Method
The detection of 1-hydroxycyclohexyl phenyl ketone in aqueous injection solutions is critical due to its potential cytotoxic effects. In this study, gas chromatography-mass spectrometry, commonly referred to as GC-MS, was employed to analyze the levels of 1-hydroxycyclohexyl phenyl ketone. A systematic sample extraction process was carried out, where a liquid-phase extraction technique was used to isolate the photoinitiator from the samples. Following extraction, the solvent was evaporated under a stream of nitrogen at 50 degrees Celsius, yielding a residue that was subsequently dissolved in n-hexane. This dissolved sample was then injected into the GC-MS system for quantification. The analysis revealed that the concentration of 1-hydroxycyclohexyl phenyl ketone in the aqueous solutions ranged from 6.13 to 8.32 micrograms per milliliter in 20 milliliter vials, underscoring the relevance of this detection method in identifying potential contaminants in pharmaceutical preparations. 1
Implications of 1-Hydroxycyclohexyl Phenyl Ketone Detection
The quantification of 1-hydroxycyclohexyl phenyl ketone not only serves to highlight its presence in aqueous solutions but also raises concerns regarding its cytotoxicity to human cells. Utilizing the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay for cell viability, researchers treated normal human peripheral blood mononuclear cells with 1-hydroxycyclohexyl phenyl ketone for both 24 and 48 hours at 37 degrees Celsius. Results indicated a significant decrease in cell viability, pointing to the harmful effects of this photoinitiator on human monocytes. The findings emphasize the need for careful monitoring of 1-hydroxycyclohexyl phenyl ketone levels in clinical settings, as accumulation of this compound could potentially lead to adverse health outcomes in patients receiving intravenous treatments. Further studies are necessary to fully elucidate the health risks associated with 1-hydroxycyclohexyl phenyl ketone exposure. 2
Reference
1. Lu Y, Tan WY, Ding Y, Chen W, Zhang H. Oxidation of Primary Alcohols and Aldehydes to Carboxylic Acids with 1-Hydroxycyclohexyl Phenyl Ketone. J Org Chem. 2023; 88(13): 8114-8122.
2. Yamaji K, Kawasaki Y, Yoshitome K, Matsunaga H, Sendo T. Quantitation and human monocyte cytotoxicity of the polymerization agent 1-hydroxycyclohexyl phenyl ketone (Irgacure 184) from three brands of aqueous injection solution. Biol Pharm Bull. 2012; 35(10): 1821-1825.
- Related articles
- Related Qustion
Zinc acetate regulates copper absorption by inducing metallothionein production, helping manage Wilson's disease safely and effectively, even during pregnancy.....
Sep 10,2024API25-hydroxycholesterol can be generated by both enzymatic and nonenzymatic pathways. The enzyme responsible for 25-HC biogenesis is mainly the 25-hydroxylase.....
Sep 10,2024Biochemical Engineering1-Hydroxycyclohexyl phenyl ketone
947-19-3You may like
1-Hydroxycyclohexyl phenyl ketone manufacturers
- 1-Hydroxycyclohexyl phenyl ketone
-

- $5.00 / 25kg
- 2024-09-10
- CAS:947-19-3
- Min. Order: 1kg
- Purity: >99%
- Supply Ability: 200mt/year
- 1-Hydroxycyclohexyl phenyl ketone
-

- $0.00 / 1KG
- 2024-09-06
- CAS:947-19-3
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month
- 1-Hydroxycyclohexyl phenyl ketone
-

- $2.00 / 1KG
- 2024-08-23
- CAS:947-19-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 ton