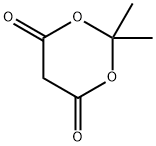
2,2-Dimethyl-1,3-dioxane-4,6-dione: History and Versatility in Organic Synthesis
Sep 25,2024
General Description
2,2-Dimethyl-1,3-dioxane-4,6-dione, known as Meldrum's acid, originates from Andrew Norman Meldrum's early 20th-century research, where he synthesized it using a reaction of acetone and malonic acid. Although its structure was clarified in 1948, the compound gained recognition for its unique C–H acidity and versatility in organic synthesis. Over the decades, particularly since the 1980s, 2,2-Dimethyl-1,3-dioxane-4,6-dione has been pivotal in multicomponent reactions and the synthesis of heterocycles, including spiro-pyrimidine compounds. Today, its applications continue to expand, demonstrating its significance in advancing organic chemistry methodologies and synthetic strategies.

Figure 1. 2,2-Dimethyl-1,3-dioxane-4,6-dione
History
Legacy of Andrew Norman Meldrum
The history of 2,2-Dimethyl-1,3-dioxane-4,6-dione, more famously known as Meldrum's acid, traces back to the pioneering work of Scottish chemist Andrew Norman Meldrum in the early 20th century. Meldrum, born in 1876, laid the groundwork for synthesizing this compound through his meticulous research. In his first independent publication, he investigated the reaction between acetone and malonic acid, employing a novel mixture of acetic anhydride and sulfuric acid as a condensing agent. The resulting compound, despite initial structural uncertainties, hinted at the potential of 2,2-Dimethyl-1,3-dioxane-4,6-dione, although its true structure was not fully elucidated until later in 1948. This compound has since emerged as an invaluable reagent in the synthesis of heterocycles and various organic reactions, showcasing its versatility and significance in organic chemistry. 1
Advancements and Recognition
The journey of 2,2-Dimethyl-1,3-dioxane-4,6-dione continued well into the following decades, especially following the determination of its correct structure. Subsequent research highlighted the unique properties of this compound, particularly its unusual C–H acidity, which has been a subject of ongoing investigation. The interest in 2,2-Dimethyl-1,3-dioxane-4,6-dione surged throughout the 1980s and has remained strong into the 21st century, as evidenced by numerous reviews focusing on its applications, including in multicomponent reactions and the synthesis of natural products. Despite Andrew Norman Meldrum's humble legacy, the contributions of 2,2-Dimethyl-1,3-dioxane-4,6-dione in the field of chemistry have solidified its reputation, with many researchers acknowledging the enduring significance of this compound in promoting advancements in organic chemistry and synthetic methodologies. 1
Versatility in Organic Synthesis
2,2-Dimethyl-1,3-dioxane-4,6-dione is increasingly recognized for its unique role as a one-carbon synthon in various organic syntheses, particularly in the formation of complex heterocycles. When 2,2-Dimethyl-1,3-dioxane-4,6-dione participates in reactions, the added carbon originates from its CH₂ group, proving crucial for building intricate molecular architectures. Notably, 2,2-Dimethyl-1,3-dioxane-4,6-dione retains its 1,3-benzodioxane structure through several heterocycle-forming reactions, facilitating diverse synthetic pathways. For instance, in a Biginelli-like three-component reaction involving benzaldehydes and urea, spiro-pyrimidine compounds are formed instead of the expected intermediates, underscoring the versatility of 2,2-Dimethyl-1,3-dioxane-4,6-dione in creating novel chemical entities. 2
Mechanistic Studies and Reaction Pathways
The mechanisms by which 2,2-Dimethyl-1,3-dioxane-4,6-dione functions as a C1-synthon are complex and often depend on the substituents present on the reacting aldehydes. In reactions where electron-withdrawing para-substituted benzaldehydes are utilized, the pathway is favored toward spiro-product formation. Conversely, reactions with para-electron-donating groups tend to yield Knoevenagel adducts. This differentiation in outcomes can be attributed to a proposed cascade mechanism that facilitates either Knoevenagel condensation or a reaction involving urea. Such insights into the reactivity of 2,2-Dimethyl-1,3-dioxane-4,6-dione have led researchers to explore various catalysts and conditions, thus driving advancements in synthetic methodologies employing this compound. 2
Broad Applications in Synthesis
The utility of 2,2-Dimethyl-1,3-dioxane-4,6-dione extends beyond simple transformations; it serves as a pivotal component in constructing more complex frameworks such as spiro-complexes and pyridine derivatives. The interplay between 2,2-Dimethyl-1,3-dioxane-4,6-dione and various electrophiles in tandem reactions demonstrates its capability to generate multiple C–C bonds in a single step, significantly simplifying synthetic routes. Notably, the aza Diels–Alder reaction of 2,2-Dimethyl-1,3-dioxane-4,6-dione-derived oxyamines showcases its effectiveness in yielding highly substituted pyridine compounds with impressive yields when catalyzed appropriately. Despite the remarkable reactivity exhibited by 2,2-Dimethyl-1,3-dioxane-4,6-dione, the full scope of its applications in heterocyclic synthesis is still being explored, highlighting its importance as a versatile building block in contemporary organic chemistry. 2
References:
[1] VICTORIA V. LIPSON N Yu G. One hundred years of Meldrum’s acid: advances in the synthesis of pyridine and pyrimidine derivatives[J]. Molecular Diversity, 2009, 13 4. DOI:10.1007/s11030-009-9136-x.
[2] LISA ZEUSSEL S S. Meldrum’s Acid Furfural Conjugate MAFC: A New Entry as Chromogenic Sensor for Specific Amine Identification.[J]. Molecules, 2023, 28 18. DOI:10.3390/molecules28186627.
- Related articles
- Related Qustion
- Synthesis methods of 2,2-Dimethyl-1,3-dioxane-4,6-dione Jun 14, 2022
2,2-Dimethyl-1,3-dioxane-4,6-dione is an organic compound, which is a white solid at room temperature and decomposes when heated.
- Application and toxicological of 2,2-Dimethyl-1,3-dioxane-4,6-dione Apr 12, 2022
Meldrum’s acid (2,2-dimethyl-1,3-dioxane-4,6-dione; isopro-pylidene malonate) is an organic compound, discovered in 1908 by A. N. Meldrum.1 Meldrum misidentified the structure as b-lactone with carboxylic acid group at position, and the cor
- Uses and Synthesis of Meldrum’s acid Mar 22, 2022
Meldrum’s acid has attracted considerable attention due to its high acidity (pKa = 4.97) and rigid cyclic structure. Acylated derivatives (synthetic equivalents of mixed ketenes) readily undergo alcoholysis to give b-keto esters. Alkylidene
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringEmtricitabine(FTC) is a nucleoside reverse transcriptase inhibitor (NRTI) used in combination with other antiretroviral therapies for treating HIV-1, HIV-2, and HBV.....
Sep 25,2024Drugs2,2-Dimethyl-1,3-dioxane-4,6-dione
2033-24-1You may like
2,2-Dimethyl-1,3-dioxane-4,6-dione manufacturers
- 2,2-Dimethyl-1,3-dioxane-4,6-dione
-

- $1000.00 / 1kg
- 2025-12-16
- CAS:2033-24-1
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- 2,2-dimethyl-1,3-dioxane-4,6-dione; Meldrum's acid; Isopropylidene malonate
-
- $36.00 / 1kg
- 2025-12-16
- CAS:2033-24-1
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 100kg
- 2,2-Dimethyl-1,3-dioxane-4,6-dione
-

- $0.00 / 25KG
- 2025-12-16
- CAS:2033-24-1
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month