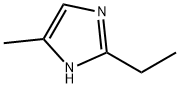
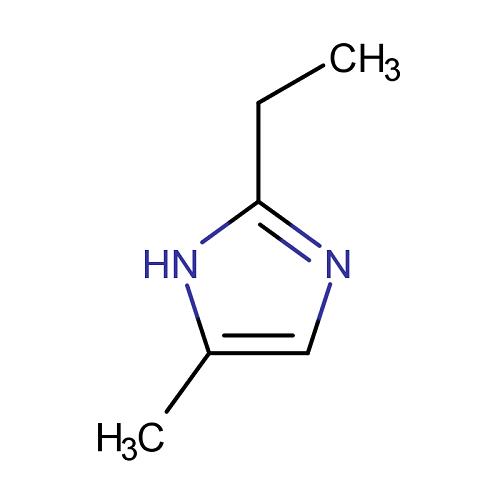
2-ethyl-4-methylimidazole: a comprehensive analysis from chemical structure to industrial applications
Oct 22,2024
Introduction
2-Ethyl-4-methylimidazole (EMI-24) is an imidazole compound that is widely used as a curing agent in a variety of epoxy resin systems. EMI-24 is also used as a synthesis intermediate and as a base material for the preparation of other active ingredients. In addition, EMI-24 can also be used in industry as a promising low-cost carbon dioxide absorber.

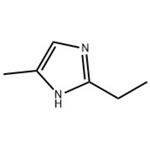
Chemical structure of 2-ethyl-4-methylimidazole
2-Ethyl-4-methylimidazole appears as a colourless to slightly yellow liquid with an amine-like odour. It has good compatibility with epoxy resin and is a key raw material for the preparation of high performance curing agents. It is also a widely used intermediate.
Synthesis
The method for the preparation of 2-Ethyl-4-methylimidazole comprises the following steps: diamine and propionitrile are catalyzed by a catalyst to carry out a cyclization reaction at a temperature of 80-110 °C and 120-140 °C in sequence; the product after the cyclization reaction is dehydrogenated by rhenium nickel at a temperature of 170-200 °C to obtain 2-Ethyl-4-methylimidazole. The preparation method of 2-ethyl-4-methylimidazole adopts step-by-step method, which has the advantages of reducing process cost, optimising the reaction process, simple and convenient operation, reducing pollution and high reaction yield.
Uses
2-Ethyl-4-methylimidazole is used as a cross-linking agent in epoxy resins for the synthesis of network polymers with higher heat resistance and physical properties. It is also particularly well suited for efficient carbon dioxide capture. 2-Ethyl-4-methylimidazole in aqueous solution has a maximum molar CO2 uptake capacity of 1.0 mol∙mol-1 and the absorbed CO2 is fully released by heating the solution at relatively low temperatures (<100 °C). Stability tests show that the aqueous system is quite stable, with less than 10% loss of molar absorptive capacity after eight absorption-desorption cycles. Time-dependent in situ decaying total reflectance infrared absorption spectroscopy and 13C nuclear magnetic resonance spectroscopy studies showed that the intermediate products in the CO2 absorption-desorption process were HCO3- and H2CO3. These intermediate products decomposed easily, which was responsible for the low CO2 desorption temperatures and high desorption efficiency of the system. In addition, 2-ethyl-4-methylimidazole aqueous solution separates and purifies carbon dioxide from flue gas and even ambient air. Thus, 2-ethyl-4-methylimidazole is a promising low-cost carbon dioxide absorber for industrial use.
Mechanism
In the study of crosslinked epoxyimidazole systems, in particular the diglycidyl ether of bisphenol A (DGEBA)/EMI-24 system, it has been found that the crosslinking system is more complex than the PGE-based system. adduct formation is the major reaction and consumes most of the epoxide groups. Alternatively, at 25.0 mole% EMI-24, only one-half of the epoxide groups are used by the imidazole in forming adducts, and the remaining epoxide groups can then react to form ether crosslinks. The second exothermic peak in the 25.0 mole% thermogram can be attributed to this etherification reaction. This mechanism also accounts for the increase in the Tg of the cured samples as the EMI-24 concentration decreases from 50.0 to 25.0 mole%. This would indicate that the amine-epoxide reaction is necessary before the etherification reaction can proceed. Since the adduct formation is not a crosslinking reaction, the Tg of the sample will not change dramatically until there is significant formation of ether groups.

References:
[1] M. S. HEISE G C M. Mechanism of 2-ethyl-4-methylimidazole in the curing of the diglycidyl ether of bisphenol A[J]. Journal of Polymer Science Part C: Polymer Letters, 1988, 26 3: 129-173. DOI:10.1002/pol.1988.140260305.
[2] XINGTIAN ZHANG. Aqueous 2-Ethyl-4-methylimidazole Solution for Efficient CO2 Separation and Purification[J]. Separations, 2023. DOI:10.3390/separations10040236.
- Related articles
- Related Qustion
- Synthesis and Application of 2-ethyl-4-methylimidazole Jul 21, 2022
2-ethyl-4-methylimidazole is an excellent curing agent, used in the preparation of epoxy adhesive epoxy silicone coating.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIThis review delves deep into 4-Cyanophenol molecular structure, diverse applications, environmental implications, and the current landscape of research.....
Oct 21,2024API2-Ethyl-4-methylimidazole
931-36-2You may like
2-Ethyl-4-methylimidazole manufacturers
- 2-Ethyl-4-methylimidazole
-
- 2025-10-27
- CAS:931-36-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- 2-Ethyl-4-methylimidazole
-

- $0.00 / 200Kg/Drum
- 2025-10-27
- CAS:931-36-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 2000mt/year
- 2-Ethyl-4-methylimidazole
-
- $0.00 / 100g
- 2025-10-24
- CAS:931-36-2
- Min. Order: 100g
- Purity: 96%
- Supply Ability: 500kg/M