4-Bromo-2,5-difluorobenzoic Acid: Pharmaceutical Applications and Synthesis Method
Apr 16,2024
General Description
4-Bromo-2,5-difluorobenzoic acid exhibits significant potential in pharmaceutical applications, especially as a modulator of dopamine neurotransmission and a dopaminergic stabilizer. It belongs to a unique class of derivatives known as 1-(4H-1,3-benzodioxin-2-yl)methanamine derivatives. 4-Bromo-2,5-difluorobenzoic acid has shown promise in treating central nervous system disorders, including Alzheimer's disease, due to its ideal drug half-life and efficacy. Moreover, 4-Bromo-2,5-difluorobenzoic acid demonstrates anti-cancer properties through its role in the synthesis of 5-oxo-4,5-dihydro-1H-1,2,4-triazole derivatives, which have proven effective in combating hyperproliferative diseases like cancer. Further research is essential to fully explore the therapeutic potential of 4-Bromo-2,5-difluorobenzoic acid in pharmaceuticals. The synthesis method outlined provides a pathway to obtain this compound, paving the way for its utilization in various medicinal applications, highlighting its importance in drug development and disease treatment.

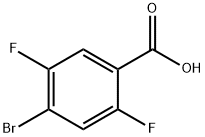
Figure 1. 4-Bromo-2,5-difluorobenzoic acid
Pharmaceutical Applications
4-Bromo-2,5-difluorobenzoic acid has shown significant potential in pharmaceutical applications. Its pharmacological properties make it suitable for various medical uses. One notable application of 4-Bromo-2,5-difluorobenzoic acid lies in its role as a modulator of dopamine neurotransmission, specifically as a dopaminergic stabilizer. This compound belongs to a novel class of derivatives called 1-(4H-1,3-benzodioxin-2-yl)methanamine derivatives. It exhibits remarkable activity in treating central nervous system (CNS) disorders. The compound has been investigated for its therapeutic effects in CNS disorders, such as Alzheimer's disease. Compared to existing compounds, 4-Bromo-2,5-difluorobenzoic acid demonstrates an ideal drug half-life and excellent efficacy. It has the potential to be an effective treatment option for diseases associated with Alzheimer's disease. Furthermore, research has shown that 4-Bromo-2,5-difluorobenzoic acid possesses anti-cancer properties. It can be used in the preparation of 5-oxo-4,5-dihydro-1H-1,2,4-triazole derivatives, which have demonstrated efficacy in the treatment of hyperproliferative diseases, particularly cancer. These derivatives have been evaluated in enzymatic and proliferation assays, showcasing their potential as anti-cancer agents. In conclusion, 4-Bromo-2,5-difluorobenzoic acid holds promise in the field of pharmaceuticals due to its ability to modulate dopamine neurotransmission and its potential applications in treating CNS disorders, notably Alzheimer's disease. Additionally, its role in the synthesis of 5-oxo-4,5-dihydro-1H-1,2,4-triazole derivatives highlights its potential as an anti-cancer agent. Further research and development are needed to fully explore the therapeutic possibilities of this compound. 1,2
Synthesis Method
The synthesis of 4-Bromo-2,5-difluorobenzoic acid involves a series of key steps to achieve its production. The process begins by preparing a solution of 1,4-dibromo-2,5-difluorobenzene in dry 1,2-dichloroethane under a nitrogen atmosphere at a low temperature of -78°C. To this solution, n-butyllithium is added dropwise at the same low temperature. The reaction mixture is then stirred for a specific duration before being quenched with an excess of solid CO2. Afterwards, the mixture is allowed to warm up to room temperature, and water is introduced to aid in the separation of phases. The organic layer is then subjected to multiple extraction steps using a 10% Na2CO3 solution and ethyl acetate, which helps isolate the desired compound. To further process the organic layers, they are dried with Na2SO4, followed by filtration and concentration under vacuum. This process ultimately leads to the formation of 4-Bromo-2,5-difluorobenzoic acid as the final product. The significance of this synthetic route lies in the potential applications of 4-Bromo-2,5-difluorobenzoic acid. It serves as a crucial modulator of dopamine neurotransmission, specifically functioning as a dopaminergic stabilizer. Its therapeutic potential extends to the treatment of central nervous system (CNS) disorders. Additionally, the compound belongs to a unique class of derivatives known as 1-(4H-1,3-benzodioxin-2-yl)methanamine derivatives, which adds to its importance in the field of medicinal chemistry. In summary, the synthesis of 4-Bromo-2,5-difluorobenzoic acid involves the preparation of a solution, addition of n-butyllithium, quenching, phase separation, extraction, drying, filtration, and concentration. This synthetic route is significant due to the compound's role as a dopaminergic stabilizer and its potential therapeutic applications in CNS disorders. Its classification as a 1-(4H-1,3-benzodioxin-2-yl)methanamine derivative further enhances its relevance in medicinal chemistry. 3
Reference
1. Gradl SN, Niehues M, Halfbrodt WG. Preparation of 5-oxo-4,5-dihydro-1H-1,2,4-triazole derivatives for the treatment of cancer. 2019; Patent Number: EP3553052.
2. Chen L, Zhao J. Preparation of the delta opioid receptor antagonist and their medical application. 2019; Patent Number: CN109896991.
3. Sonesson C, Svensson P, Andersson M. Preparation of 1-(4H-1,3-benzodioxin-2-yl)methanamine derivatives as modulators of dopamine neurotransmission for treating CNS disorders and other conditions. 2009; Patent Number: WO2009133110.
- Related articles
- Related Qustion
- 4-bromo-2,5-difluorobenzoic acid: Preparation and Applications in Bioorganic Chemistry Mar 15, 2023
4-Bromo-2,5-difluorobenzoic acid is a synthetic chemical compound that is widely used in scientific research.
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APINADP, Disodium Salt is the sodium salt form of NADP. NADP, Disodium Salt is a white to pale yellow amorphous powder.....
Apr 16,2024Biochemical Engineering4-bromo-2,5-difluorobenzoic acid
28314-82-1You may like
4-bromo-2,5-difluorobenzoic acid manufacturers
- 4-bromo-2,5-difluorobenzoic acid
-

- $10.00 / 1KG
- 2025-03-31
- CAS:28314-82-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4-Bromo-2,5-difluorobenzoic acid
-

- $100.00 / 1kg
- 2024-04-24
- CAS:28314-82-1
- Min. Order: 1kg
- Purity: 99.93%
- Supply Ability: 1000kg per week
- 4-bromo-2,5-difluorobenzoic acid
-

- $1.00 / 1KG
- 2019-07-12
- CAS:28314-82-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg