4'-Bromo-3'-methylacetophenone:Synthesis,Applications
Mar 6,2023
General Description
4'-Bromo-3'-methylacetophenone,with the CAS number 37074-40-1, is a versatile building block that is used in the synthesis of many complex compounds. It can be used as a reactant, reagent, or speciality chemical. 4-Bromo-3-methylbenzaldehyde is an intermediate for the production of other chemicals and has been shown to be useful in the synthesis of various scaffolds.

Figure 1. Properties of 4'-Bromo-3'-methylacetophenone
Synthesis
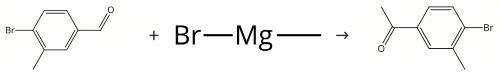
Figure 2. Synthesis of 4'-Bromo-3'-methylacetophenone
A solution of 4-bromo-3-methylbenzaldehyde (4 g, 20 mmol) in dry tetrahydrofuran (40 mL) was cooled to 0°C under nitrogen atmosphere, and then methyl magnesium bromide (20 mL, 1N in tetrahydrofuran) was added dropwise. The ice bath was removed, and the mixture was stirred for 2 hours. Ammonium chloride aqueous (40 mL) was added and the mixture was extracted with dichloromethane (20 mL * 3). The organic phase was dried by sodium sulphate, filtered and concentrated to give a residue. The residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 5:1) to give 1-(4-bromo-3-methylphenyl)ethanol. Colorless oil, yield 4.0 g, 93%. Pyridinium chlorochromate (48 g, 223 mmol) was added to a solution of 1-(4-bromo-3-methylphenyl)ethanol (31.9 g, 148 mmol) in dichloromethane (800 mL). The mixture was stirred at room temperature for 2 hours. The mixture was concentrated to give a residue. The residue was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 25:1) to give 4'-Bromo-3'-methylacetophenone. Yield 27.3 g, 87%.1
Applications of 4'-Bromo-3'-methylacetophenone
Intermediate in preparation of biphenyls and analogs
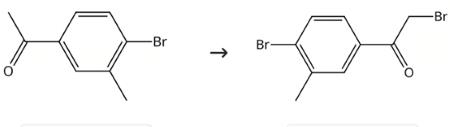
Figure 3. Intermediate in preparation of biphenyls and analogs
Trimethyl phenylammonium tribromide (13 g, 0.036 mol) was added to a solution of 4'-Bromo-3'-methylacetophenone (6.3 g, 0.030 mol) in THF (30 mL) and the reaction mixture was stirred at RT for 12 h, filtered, and concentrated. The title intermediate (8.6 g, 100% yield).2
Intermediate in preparation of isoxazoline derivatives
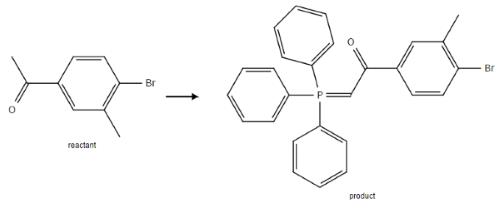
Figure 4. Intermediate in preparation of isoxazoline derivatives
1300 mg of 4'-Bromo-3'-methylacetophenone (Vila) (Journal of Organic Chemistry (1947), 12, 617-703) was stirred with 1300 mg of triphenylphosphine in 20 ml of dichloromethane at ambient temperature for 18 hours. The mixture was diluted with toluene and the separated phosphonium salt was filtered off. It was then suspended in 20 ml of dichloromethane and 20 ml of water. 800 mg (7.55 mmol) of sodium bicarbonate was then added in 20 ml of water and the mixture was stirred overnight. The organic layer was separated, dried and evaporated under reduced pressure. The residue was triturated with hexane giving 1.6 g (86%) of 1-(4-Bromo-3-methyl-phenyl)-2-(triphenylphosphanylidene)-ethanone (Va) as a solid.3
Intermediate in preparation of substituted 6-(2-tolyl)-triazolo[1,5-a]pyrimidines as fungicides
![Intermediate in preparation of substituted 6-(2-tolyl)-triazolo[1,5-a]pyrimidines as fungicides.png Intermediate in preparation of substituted 6-(2-tolyl)-triazolo[1,5-a]pyrimidines as fungicides.png](/NewsImg/2023-02-14/6381198297211194378764392.jpg)
Figure 5. Intermediate in preparation of substituted 6-(2-tolyl)-triazolo[1,5-a]pyrimidines as fungicides
To a solution of 4'-Bromo-3'-methylacetophenone (2mmol) in ethanol was added dimethyl amine (5 equiv.), paraformaldehyde (5 equiv.) and aq. HCl After 12h, the solvent was removed and ethyl acetate and 1N NaOH were added and organic layer was extracted 3 times. It was dried over MgSO4, filtered, evaporated and the filtrate was concentrated at reduced pressure. The residue was purified by column chromatography eluting with ethyl acetate and hexane. The product was dissolved in methanol and added NaBH4 (2 equiv.). After 2h, sat. NaHCO3 and ethyl acetate were added and the organic layer was extracted 3 times with ethyl acetate. It was dried over MgSO4, filtered, evaporated and the filtrate was concentrated at reduced pressure. The residue was purified by column chromatography eluting with ethyl acetate and methanoL The resulting residue was dissolved in THF and was added 1H-tetrazole (1.5equiv.), triphenylphosphine (1.5equiv.) and diisopropyl azodicarboxylate (1.5equiv.) dropwise at 0°C and warmed to room temperature. After 2h, the solvent was removed and the residue was purified by column chromatography eluting with ethyl acetate and methanol. [3-(4-Bromo-3-methyl-phenyl)-3-tetrazol-2-yl-propyl]-dimethyl-amine.4
References
1. World Intellectual Property Organization, WO2013075083 A1 2013-05-23
2. World Intellectual Property Organization, WO2012061552 A1 2012-05-10
3. World Intellectual Property Organization, WO2011101402 A1 2011-08-25
4. World Intellectual Property Organization, WO2009148290 A2 2009-12-10
- Related articles
- Related Qustion
- Unveiling the Versatility of 4'-Bromo-3'-methylacetophenone: A Catalyst for Innovation in Chemical Synthesis and Application May 8, 2024
4'-Bromo-3'-methylacetophenone, a notable compound in the realm of organic chemistry, exemplifies versatility and efficacy.
The metformin is biguanide containing two coupled molecules of guanidine with additional substitutions. It has been widely used to treat diabetes since the 1950s.....
Mar 3,2023APIStannous octoate is a yellow to white viscous liquid. It is often used as an auxiliary agent for polyurethane industry, as well as an efficient catalyst and antioxidant.....
Mar 6,2023API4'-Bromo-3'-methylacetophenone
37074-40-1You may like
- Sorbic acid: Benefits and Side effects
Jul 19, 2024
- Uses of Sulfanilic acid
Jul 19, 2024
- Sodium Lauryl Ether Sulfate: Applic....
Jul 19, 2024
4'-Bromo-3'-methylacetophenone manufacturers
- 4'-Bromo-3'-methylacetophenone
-

- $100.00 / 1kg
- 2024-04-24
- CAS:37074-40-1
- Min. Order: 1kg
- Purity: 99.93%
- Supply Ability: 1000kg per week
- 4'-Bromo-3'-methylacetophenone
-

- $10.00 / 1KG
- 2023-05-05
- CAS:37074-40-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 4'-Bromo-3'-methylacetophenone
-

- $1.00 / 1KG
- 2019-07-14
- CAS:37074-40-1
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100kg





