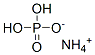Ammonium dihydrogen phosphate: a multifunctional inorganic compound and its chemical properties
Oct 23,2024
Ammonium dihydrogen phosphate is a colourless or white crystals, used as buffering agent, dough conditioner, leavening agent.

Chemical properties
Ammonium dihydrogen phosphate is soluble in water and crystallizes from it as the anhydrous salt in the tetragonal system, as elongated prisms or needles. It is practically insoluble in ethanol.
Solid ammonium dihydrogen phosphate can be considered stable in practice for temperatures up to 200 °C, when it decomposes into gaseous ammonia NH3 and molten phosphoric acid H3PO4. At 125 °C the partial pressure of ammonia is 0.05 mm Hg.1
Applications
Agriculture
The largest use of monoammonium phosphate by weight is in agriculture, as an ingredient of fertilizers. It supplies soil with the elements nitrogen and phosphorus in a form usable by plants. Its NPK label is 12-61-0 (12-27-0), meaning that it contains 12% by weight of elemental nitrogen and (nominally) 61% of phosphorus pentoxide P2O5, or 27% of elemental phosphorus.
Fire extinguishers
The compound is also a component of the ABC powder in some dry chemical fire extinguishers.
Method of assay
Dissolve about 500 mg of the sample, accurately weighed, in 50 ml of water, and titrate to a pH of 8.0 with 0.1 N sodium hydroxide. Each ml of 0.1 N sodium hydroxide is equivalent to 11.50 mg of Ammonium dihydrogen phosphate.
1.Dejun Xu, Xing Xiong, Lin Yang, Zhiye Zhang, and Xinlong Wang (2016): "Determination of the Solubility of Ammonium Dihydrogen Phosphate in Water-Ethanol System at Different Temperatures from 283.2 to 343.2 K".Journal of Chemincal Engineering Data, volume 61, issue 1, pages 78–82.
References:
[1] DEJUN XU. Determination of the Solubility of Ammonium Dihydrogen Phosphate in Water–Ethanol System at Different Temperatures from 283.2 to 343.2 K[J]. Journal of Chemical & Engineering Data, 2015, 61 1: 1-700. DOI:10.1021/acs.jced.5b00224.
- Related articles
- Related Qustion
- Unique properties of ammonium dihydrogen phosphat Jun 27, 2022
Ammonium dihydrogen phosphate, a chemical agent, also known as monoammonium phosphate, is a white crystal with the chemical formula of NH4H2PO4.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringThis paper aims to comprehensively review the properties, preparation methods, application fields, and future development trends of zinc sulfate.....
Jul 2,2024APIAmmonium dihydrogen phosphate
7722-76-1You may like
Ammonium dihydrogen phosphate manufacturers
- Ammonium dihydrogen orthophosphate
-

- 2025-11-17
- CAS:7722-76-1
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Ammonium dihydrogen phosphate
-

- $980.00/ ton
- 2025-11-17
- CAS:7722-76-1
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 5000
- Ammonium dihydrogen phosphate
-

- $0.00 / 1KG
- 2025-11-17
- CAS:7722-76-1
- Min. Order: 1KG
- Purity: 98%min
- Supply Ability: 30tons/month