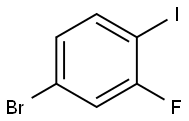
Application of 1-Bromo-3-Fluoro-4-Iodobenzene
Aug 15,2022
General description
1-Bromo-3-Fluoro-4-Iodobenzene can be used for the preparation of 2 - (4 - [4 - (3, 5 - two fluorine - 4 - (3 methyl) phenyl] - 3 - fluorinated phenyl] cyclohexanol - 3 - ene - 1 - base] - 5 - propyl - 1, 3 - dioxane, LCD of the compounds is suitable for medium, especially for example for TN the STN IPS FFS and TN-TFT displays.
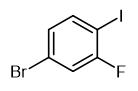
Fig. 1 The structure of 1-Bromo-3-Fluoro-4-Iodobenzene.
Physicochemical property
1-Bromo-3-Fluoro-4-Iodobenzene is a beige crystal with a melting point of 48-51 °C. Its boiling point is expected to be 243.9±20.0 °C.
Application
Preparation of liquid marble
Thermoresponsive liquid marbles were prepared from water and 1-bromo-3-fluoro-4-iodobenzene (BFI) powder with a melting point of 46.5 degrees C. The liquid marble can be floated on the surface of bulk water at room temperature, which is lower than the melting point of BFI. When the temperature of bulk water was increased above the melting point, the liquid marble disintegrated to leak the interior water to bulk water. Heating can be applied as a trigger of a chemical reaction between the interior reactant and another reactant in bulk water [1].
All-Small-Molecule Organic Solar Cells
Low cost, high efficiency, and high stability are the three key issues of organic solar cells (OSCs) that should be carefully considered to meet the requirement of future commercial applications. Therefore, the development of high-performance organic photovoltaic materials with low synthetic cost has been becoming a crucial challenge in the field of OSCs. Herein, two new low-cost small-molecule donors (SM-BF1 and SM-BF2) are designed and synthesized with a facile synthetic route by replacing 4-bromo-2-fluorobenzenethiol and 4-bromo-3-fluorobenzenethiol with low-cost 4-bromo-2-fluoro-1-iodobenzene and 4-bromo-3-fluoro-1-iodobenzene as key raw materials. Besides, the influence of the chemical steric effect of the phenyl conjugated side chains of the benzodithiophene (BDT) unit on photophysical properties, charge transfer, and photovoltaic properties are deeply investigated by the modulation of fluorine atom substituted position. As a result, SM-BF1 with ortho-fluorinated substituent has outstanding crystallization properties and better miscibility with acceptor Y6 and exhibits more desirable morphology and more balanced charge-carrier transport properties, leading to a superior power conversion efficiency (PCE) to 15.71%. More encouragingly, according to the figure of merit (FOM) and the industrial figure of merit (i-FOM) to evaluate the small-molecule donors, the SM-BF1-based device has excellent potential for future commercial applications [2].
Preparation of 3,7-dibromo-10H-phenoxazine
Preparation of 3,7-dibromo-10H-phenoxazine (III) involves dissolving 4-bromo-2-methoxyaniline, 1-bromo-3-fluoro-4-iodobenzene in toluene in the ratio of 2:3, adding sodium tert-butoxide, palladium catalyst, carrying out coupling reaction under nitrogen atmosphere at 110 degrees C for 24 hours to obtain reaction liquid (A), subjecting the reaction liquid (A) to spin-drying and purifying using petroleum ether to obtain (4-bromo-2-fluoro-phenyl)-(4- bromo-2-methoxy-phenyl)-amine intermediate product (I), dissolving intermediate product (I) in dichloromethane to obtain a reaction solution (B), adding boron tribromide solution to the reaction in drops to obtain 5-bromo-2-(-4-bromo-2-fluoro-phenylamino)-phenol intermediate product (II), adding intermediate product (II) to potassium carbonate and dimethylformamide solution under nitrogen atmosphere, heating at 120 degrees C for overnight, cooling to room temperature, extracting using ethyl acetate and purifying using column chromatography [3].
Synthesis of 2-halo-1-(4-bromo-3-fluorophenyl)ethenone
In the present invention, the α-halogenated 2-halogen τ- (4-mo-3-fluorophenyl) ethyl ketone is based on 4-mo-3-fluoroiodobenzene as raw material, and directly obtains the α-halogenated 2-halogen-1 -(4-bromo-3-fluorophenyl) ethyl ketone by the reaction of Weinreb amide corresponding to the halogenated acetic acid after treatment with the format reagent. The invention solves the shortcomings of the synthesis route is relatively long, the operation is complex, the post-treatment is difficult, and the amplification is not easy in the existing literature. The invention provides a kind of 4-bromo-3-fluoroiodobenzene as raw material, the synthesis route is simple, the process selection is reasonable, the raw material cost is low, the raw material is simple and easy to obtain, the operation and post-treatment are convenient, and the total yield is high. Easy to amplify and suitable for mass production, the synthesis method of α-halogenated τh -(4-mo-3-fluorophenyl) ethyl ketone can be obtained [4].
References
[1] Nakai K, Fujii S, Nakamura Y, et al. Thermoresponsive liquid marbles prepared with low melting point powder[J]. Chemistry Letters, 2015, 44(8): 1077-1079.
[2] Guo J, Qiu B, Yang D, et al. 15.71% Efficiency All‐Small‐Molecule Organic Solar Cells Based on Low‐Cost Synthesized Donor Molecules[J]. Advanced Functional Materials, 2022, 32(13): 2110159.
[3] Huang Z, Li Y. Preparation of 3,7-dibromo-10H-phenoxazine for thermally activated delayed fluorescence material intermediate involves dissolving 4-bromo-2-methoxyaniline, 1-bromo-3-fluoro-4-iodobenzene in toluene, adding palladium catalyst, and reacting[P]. Faming Zhuanli Shenqing, 110172043, 2019.
[4] Chu Y, Xu W, Zuo B. Synthesis of 2-halo-1-(4-bromo-3-fluorophenyl)ethanone involves reacting 1-Bromo-2-fluoro-4-iodobenzene with Weinreb amide and isopropyl magnesium chloride[P]. Faming Zhuanli Shenqing, 102372618, 2012.
- Related articles
- Related Qustion
2'-Deoxyuridine is used as a therapeutic agent in the treatment of allergy, cancer, infection and autoimmune diseases....
Aug 12,2022APIPolyethyleneimine (PEI), an organic polyamine polymer, is one of the most prominent examples of cationic polymers capable of gene transfection in vitro and in vivo into various cell lines and tissues.....
Aug 15,2022API4-Bromo-2-fluoro-1-iodobenzene
105931-73-5You may like
4-Bromo-2-fluoro-1-iodobenzene manufacturers
- 4-bromo-2-fluoro-1-iodobenzene
-

- $0.00 / 200kg
- 2025-04-16
- CAS:105931-73-5
- Min. Order: 20kg
- Purity: 99%
- Supply Ability: 20 tons
- 1-Bromo-3-fluoro-4-iodobenzene
-

- $0.00 / 200KG
- 2025-04-15
- CAS:105931-73-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- 1-BROMO-3-FLUORO-4-IODOBENZENE
-
- $0.00 / 1KG
- 2025-04-04
- CAS:105931-73-5
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 1ton