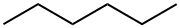Applications and Hazards of Hexane
Dec 16,2024
What is Hexane?
Hexane is a safe, efficient and widely used hydrocarbon. It has the appearance of a colourless liquid with a slightly unpleasant odour. It is highly flammable and its vapours may be explosive. Its basic physico-chemical properties are Density: 661 kg/m³, Melting point: -95.3°C, Boiling point: 68.73°C, Flash point: -22°C. The structure is shown below:
Synthesis of Hexane
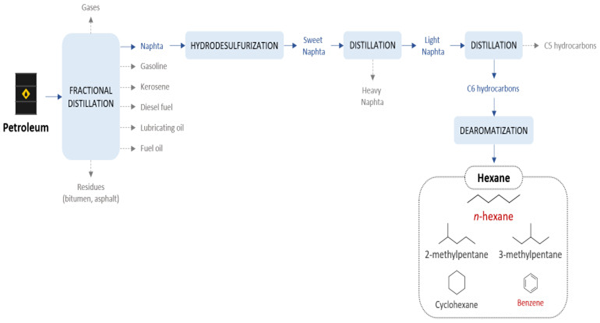
Hexane is generally produced from naphtha, one of the lightest fractions obtained directly from petroleum refining. This fraction, which makes up about 20 per cent of the weight of crude oil, consists of a mixture of C5 to C10 hydrocarbons and has a boiling point between 30 and 200 °C. It is used in a hydrodesulphurisation plant to remove sulphur and other impurities. The naphtha can be removed from sulphur and other impurities in a hydrodesulphurisation unit. This purification step is particularly necessary before catalytic reforming of the subsequent cuts, in order to avoid contamination of the metal catalysts.
The sweet naphtha obtained is further distilled into heavy naphtha and light naphtha. The former consists mainly of hydrocarbons with more than six carbon atoms and has a boiling point between 140-200 °C. The heavy naphtha is usually fed into a catalytic reformer. Heavy naphtha is usually fed into a catalytic reformer and the resulting reformate can be used directly for blending gasoline. Light naphtha, on the other hand, usually consists of hydrocarbons with six or fewer carbon atoms and has a boiling point between 30-140 °C. It is also used as a fuel for the production of gasoline. By additional distillation, the light naphtha can be further separated into a C5-rich fraction and a C6-rich fraction. Depending on the required purity, the final fraction is also de-aromatised, in order to remove aromatic impurities such as benzene.
Applications of Hexane
In the food field
Hexane is mainly used for the extraction of edible oils from seeds and vegetables, as well as for the production of flavours and fragrances, natural extracts, pharmaceuticals and nutraceuticals.
In the chemical field
Hexane is used as a special purpose solvent and cleaner. It is used as a cleaning agent in the printing, textile, furniture and shoe industries. It is also used in some speciality glues used in the roofing, shoe and leather industries. In addition, it is present in petrol, quick-drying adhesives and rubber cement. In addition, Hexane is also used as a solvent for asphalt dilution. Reducing viscosity with synthetic diluents results in a corresponding reduction in greenhouse gas emissions and significant energy savings. These results are applicable to a variety of applications, including solvent-based asphalt recycling processes, surface treatment and efficient transport of asphalt through pipelines.
In household products
Hexane is used as a liquid for cryogenic thermometers.
Hazards of Hexane
Hexane is toxic to humans. Acute (short-term) inhalation of high concentrations of hexane in humans can cause mild effects on the central nervous system (CNS), including dizziness, vertigo, mild nausea, and headache. Prolonged exposure to airborne n-hexane can result in polyneuropathy with symptoms such as numbness of the extremities, muscle weakness, blurred vision, headache and fatigue. Neurotoxic effects have also been shown in rats. However, there is no information on the carcinogenic effects of hexane in humans or animals. The US EPA has classified hexane as a Group D substance and is unable to classify it as a human carcinogen.
References:
[1] MOHAMMAD SHAH FAISAL KHAN ?, ? Density and Viscosity of Multicomponent Solvent (n-Pentane + n-Hexane + n-Heptane + cyclo-Hexane + Toluene) and Bitumen Mixtures─Implications for In Situ Bitumen Recovery and Transportation of Diluted Bitumen[J]. Energy & Fuels, 2023, 38 1: 1-742. DOI:10.1021/acs.energyfuels.3c03519.
- Related articles
- Related Qustion
- Applications of hexane as a non-polar solvent Dec 20, 2023
Hexane is undoubtedly the most widely used among the solvents used industrially for extracting non-polar edible natural products such as colors, flavors, fragrances, or lipids.
- Hexane: Preparation, Uses and Toxicities Apr 13, 2023
Hexane is an organic compound, a straight-chain alkane with six carbon atoms with the molecular formula C6H14.
- Hexane-Hazard and Toxicity Sep 6, 2019
n-Hexane is a highly flammable liquid, usually isolated from crude oil, and has extensive industrial applications as a solvent in adhesive bandage factories and other industries. It is highly toxic, triggering several adverse health effects
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringIsoamyl nitrite (amyl nitrite) is an intermediate for perfume preparation. It is an ingredient of “room odorizers.”....
Aug 5,2024Organic Chemistry