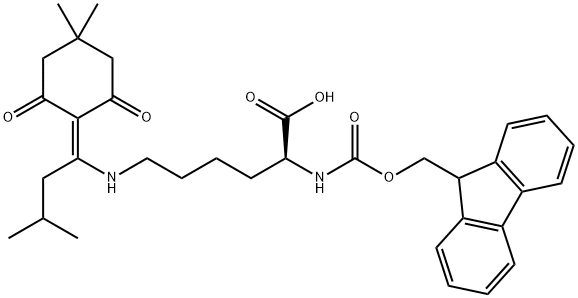
Applications of Fmoc-Lys(IVDDE)-OH
Nov 4,2019
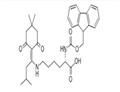
Fmoc-Lys(IVDDE)-OH is orthogonally protected lysine. The Ddiv protection is stable to piperidine.
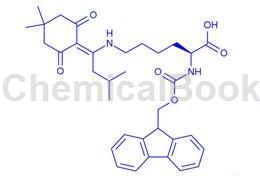
Fmoc-Lys(IVDDE)-OH is a building block to prepare peptides [1-6].
Example 1 [1]

This compound was prepared by the Fmoc chemistry-based manual SPPS on Rink Amide MBHA resin. For each amino acid coupling reaction, four equivalents of a Nα-Fmoc-protected amino acid,3.8 equivalents of the coupling reagent HBTU and the additive HOBt were used in the presence of 0.4 M NMM/DMF, and the coupling reaction was allowed to proceed at room temperature for 1 h. A 20 percent (v/v) piperidine/DMF solution was used for Fmoc removal. After the completion of the on-resin amino acid assembling and the N-terminal -amino group acetylation with acetic anhydride in the presence of 0.4 M NMM/DMF, the side chain Mtt protecting group from two Lys(Mtt) residues were selectively removed with a 1 percent (v/v) TFA/DMF solution before the two exposed free amino groups were acylated with Fmoc-glycine under peptide coupling reaction condition. After the Fmoc removal from the two incorporated Fmoc-glycine residues with a 20 percent (v/v) piperidine/DMF solution, the two newly exposed free amino groups were then acylated with suberic acid at room temperature for 1 h under peptide coupling reaction condition.
The resulting resin-bound cyclized peptide was then treated with a 2percent (v/v) solution of hydrazine (NH2NH2) in DMF, the exposed free amino group at the central position was then reacted with ethyl 3-isothiocyanatopropionate (2 x 5 h). The subsequent treatment with reagent K (83.6 percent (v/v) TFA, 5.9 percent (v/v) phenol, 4.2percent (v/v) ddH2O, 4.2 percent (v/v) thioanisole, and 2.1 percent (v/v) ethanedithiol) at room temperature for 4 h cleaved the crude ethyl ester intermediate from the resin and removed the side chain Pbf and tBu protecting groups as well. Following the concentration of the cleavage filtrate and the precipitation in cold diethyl ether of the crude ethyl ester intermediate (34 percent pure per analysis with RP-HPLC on an analytical C18 column (0.46 x 25 cm,5 μm)), it was purified by RP-HPLC on a semi-preparative C18 column (1 x 25 cm, 5 μm).
The column was eluted with a gradient of ddH2O containing 0.05 percent (v/v) TFA (mobile phase A) and acetonitrile-containing 0.05percent (v/v) TFA (mobile phase B) (0 percent–60 percent B in 60 min) at 4.5 mL/min and monitored at 214 nm. The pooled desired HPLC fractions were concentrated in vacuo to remove acetonitrile, and the remaining aqueous solution was lyophilized to afford the purified ethyl ester intermediate in an overall synthetic yield of 41 percent as a puffy white solid whose exact mass was confirmed by a unit-resolution ESI-MS analysis. This purified intermediate was then dissolved in a mixture of MeOH/ddH2O(3/1, v/v), and to the resulting solution was added at 0 °C LiOH to a final concentration of ~12.5 M. The reaction mixture was subsequently stirred at 4 °C overnight, acidified at 0 °C with 6 N HCl to pH~1, and concentrated under reduced pressure.
The ethyl ester hydrolysis product 4 was then isolated as a puffy white solid from the resulting residue by semi-preparative RP-HPLC as described above using the following gradient of the afore-mentioned mobile phases A and B: 0 percent–40 percent B in 60 min. The purity of the purified 4 was >95percent as verified by RP-HPLC on an analytical C18 column (0.46 x 25 cm,5 μm) eluted with the following gradient of the afore-mentioned mobile phases A and B: 0 percent–30 percent B in 60 min.
Example 2 [2]

These two compounds were prepared by the Fmoc chemistry-based manual solid phase peptide synthesis (SPPS) on the Rink amide MBHA resin. For each amino acid coupling, 4 equivalents of a Nα-Fmoc-protected amino acid, 3.8 equivalents of the coupling reagent HBTU and the additive HOBt were used in the presence of 0.4 M NMM/DMF, and the coupling reaction was allowed to proce ed at room temperature for 1h. A 20 percent (v/v) piperidine/DMF solution was used for Fmoc removal. After the completion of the on-resin amino acid assembling and the N-terminal Fmoc removal, the side chain Mtt protecting group on Lys(Mtt) was selectively removed with a 1percent (v/v) TFA/DCM solution. The two exposed free amino groups on the resin were then each acylated with Fmoc-Gly-OH/HBTU/HOBt (4/3.8/3.8 equivalents) in the presence of 0.4 M NMM/DMF at room temperature for 1h, followed by the Fmoc removal from the two incorporated Fmoc-glycine residues.
The two newly exposed free amino groups were subsequently acylated with succinic acid/HBTU (1/1.9 equivalents) at room temperature for 1h in the presence of 0.4 M NMM/DMF. The resultant peptidyl-resin was treated with a 2percent (v/v) solution of hydrazine (NH2NH2) in DMF to remove the ivDde protecting group on the central Lys(ivDde) residue. The resulting free amino group was then reacted with 1-dodecyl isothiocyanate (10 equivalents) or 1-tetradecyl isothiocyanate (10 equivalents) at room temperature for 2 x 5h in the presence of 0.4 M NMM/DMF, and the peptidyl-resin thus obtained was subsequently treated with a TFA-containing solution (90percent (v/v) TFA, 5percent (v/v) DCM, 5percent (v/v) ddH2O) at room temperature for 4h to cleave compound 1 or 3 off of the resin. Technically, a cleavage mixture was filtered and the volatiles in the filtrate were blown away with a stream of nitrogen in a well-ventilated fuming hood, and from the residue crude 1 or 3 was precipitated out with cold diethyl ether. The obtained crude 1 and 3 were then each purified by reversed-phase high performance liquid chromatography (RP-HPLC) on a semi-preparative C18 column (1 x 25 cm, 5 µm).
The column was eluted with a gradient of ddH2O containing 0.05percent (v/v) TFA and acetonitrile containing 0.05percent (v/v) TFA at 4.5mL/min and was monitored at 214 nm. The pooled desired HPLC fractions were concentrated under reduced pressure to remove acetonitrile and the remaining aqueous solutions were lyophilized to afford the purified 1 and 3 both as puffy white solids. The purities of the purified 1 and 3 were >95percent based on RP-HPLC analysis on an analytical C18 column (0.46 x 25 cm, 5 mm).
References
1. Liu J, Huang Y, Zheng W. A Selective Cyclic Peptidic Human SIRT5 Inhibitor[J]. Molecules, 2016,21(9): 1217
2. Li S, Wu B, Zheng W. Cyclic tripeptide-based potent human SIRT7 inhibitors[J]. Bioorganic and Medicinal Chemistry Letters, 2019, 29(3):461-465.
- Related articles
- Related Qustion
Abemaciclib (trade names Verzenio and Verzenios) is a drug for the treatment of advanced or metastatic breast cancers. It was developed by Eli Lilly and it acts as a CDK inhibitor selective for CDK4 and CDK6.....
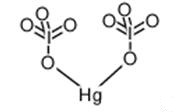
Nov 4,2019APIMercury(II) iodate(V) is dimorphous and crystallizes in two polymorphs named α- and β-Hg(IO3)2. The α-modification was prepared by precipitation of a slightly acidified Hg(NO3)2 solution with an excess of an aqueous HIO 3 solution.....
Nov 4,2019Metal halide and Halogen saltFmoc-Lys(Ddiv)-OH
204777-78-6You may like
Fmoc-Lys(Ddiv)-OH manufacturers
- Fmoc-Lys(Ddiv)-OH
-

- $0.00 / 1kg
- 2024-07-08
- CAS:204777-78-6
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1T+
- FMOC-LYS(IVDDE)-OH
-

- $8852.00 / 1KG
- 2020-06-27
- CAS: 204777-78-6
- Min. Order: 1KG
- Purity: 98.0%
- Supply Ability: 1T
- FMOC-LYS(IVDDE)-OH
-
- $1.00 / 1KG
- 2019-07-06
- CAS:204777-78-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20kg