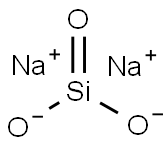Applications of Sodium Silicate
Feb 11,2022

Properties
Sodium silicates are colorless glassy or crystalline solids, or white powders. Except for the most silicon-rich ones, they are readily soluble in water, producing alkaline solutions.
Sodium silicates are stable in neutral and alkaline solutions. In acidic solutions, the silicate ions react with hydrogen ions to form silicic acids, which tend to decompose into hydrated silicon dioxide gel.Heated to drive off the water, the result is a hard translucent substance called silica gel, widely used as a desiccant. It can withstand temperatures up to 1100 °C.
Applications
Sodium silicate is made by fusing silicon dioxide (from Sand) and Sodium oxide (from Soda ash). An aqueous solution of sodium silicate is called Water glass. It forms a hard, glasslike mass when it dries. Sodium silicate has been used to preserve eggs, fireproof fabrics, and waterproof walls.
Most commonly, it is used as a cement for abrasive wheels, bonding paper, corrugated boxes and cartons, wood, glass, porcelain, leather, and textiles. A water glass solution is viscous and has little tack. For use as an adhesive, pressure must be applied to hold materials together while bonding. The dried product is brittle and water sensitive.
Aluminum salts can be added to the formulation to improve water resistance. Water glass has been used to make artificial stone. It was tried unsuccessfully as a binder in the 19th century for fresco paintings (see Mineral painting). Water glass was also used in the Ransome process of stone preservation. This procedure used alternating solutions of an alkaline silicate and Calcium chloride to form insoluble Calcium silicate in the pores of the stone.
- Related articles
- Related Qustion
- The brief introduction of Sodium silicate Apr 3, 2024
Sodium silicate is the common name for a compound sodium metasilicate, Na2SiO3, and is also referred to as liquid glass.
- Coagulation and application of Sodium silicate Dec 25, 2023
Sodium silicate is widely used as a coagulation accelerator for oil-well cement.
- Sodium Silicate - Production and Uses Nov 26, 2019
Sodium silicates are crystalline solids that have a glassy appearance. The common terms for an aqueous solution of sodium silicate are water glass and liquid glass.
Benzimidazole is a bicyclic planar, aromatic, 10π electron ring system, constituted by the fusion of a benzene ring with 4,5-positions of the imidazole ring. The nature of both the nitrogen atoms is different.....
Feb 11,2022Chemical pesticides ?Crocin is a carotenoid chemical compound that is present in the flowers crocus and gardenia. This is the chemical mainly responsible for the color of saffron. Chemically, the dieter derived from the disaccharide gentiobiose and dicarboxylic....
Feb 11,2022Biochemical EngineeringSodium silicate
1344-09-8You may like
- What is nitrogen used for?
May 24, 2024
- The structure of Aluminum oxide
May 24, 2024
- The brief introduction of Lead selenide
May 23, 2024
- Sodium silicate
-

- $6.00 / 1kg
- 2024-05-16
- CAS:1344-09-8
- Min. Order: 1kg
- Purity: More than 99%
- Supply Ability: 2000KG/Month
- Sodium silicate
-

- $18.00 / 10kg
- 2024-05-10
- CAS:1344-09-8
- Min. Order: 1kg
- Purity: 99.9
- Supply Ability: 5000
- Sodium silicate
-

- $10.00 / 1kg
- 2024-04-24
- CAS:1344-09-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000KG/Week