Apalutamide: A Promising Pharmacodynamic Drug for Prostate Cancer Treatment
Feb 6,2024
General Description
Apalutamide, a pharmacodynamic drug targeting the androgen receptor, inhibits prostate cancer cell growth and androgen-mediated gene transcription. Apalutamide affects the expression of 13 endogenous genes, leading to impaired AR function and decreased tumor volume in castration-resistant prostate cancer. At 30 mg/kg/day, it achieves a 50% reduction in tumor volume in animal models. Pharmacokinetically, Apalutamide exhibits dose-proportional increases in plasma concentration, with steady-state concentrations reached after 4 weeks. Adverse events include fatigue, hypertension, rash, and fractures, with serious AEs occurring in 24.8% of patients, emphasizing the need for close monitoring during treatment.

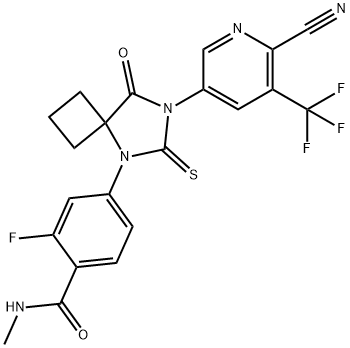
Figure 1. Apalutamide
Pharmacodynamics
Apalutamide is a pharmacodynamic drug that specifically targets the ligand-binding domain of the androgen receptor (AR). It inhibits the growth and androgen-mediated gene transcription in prostate cancer (PC) cells by directly binding to AR. This inhibition affects the expression of 13 endogenous genes, including PSA and TMPRSS2. Additionally, AR nuclear localization and DNA binding are impaired in PC cells. Studies have shown that Apalutamide has a stronger inhibitory effect on testosterone-induced expression of AR and downstream genes compared to bicalutamide in PC cells. This effect remains even under hypoxic conditions. In a murine model of castration-resistant prostate cancer (CRPC), Apalutamide demonstrates a decreased proliferative index and an increased apoptotic rate, leading to reduced tumor volume. The optimal therapeutic response is achieved at a dose of 30 mg/kg/day, where there is a 50% reduction in starting tumor volume. In male dogs, Apalutamide induces castrate-like histopathological changes in androgen-dependent reproductive organs. It also exhibits a low potential for seizures compared to enzalutamide. However, it is important to note that a missense mutation (F877L) in the ligand-binding domain of AR can confer resistance to Apalutamide and other drugs like enzalutamide. This mutation has been detected in plasma DNA of apalutamide-treated patients. 1
Pharmacokinetics
Following repeated once-daily administration, Apalutamide (30–480 mg) exhibits dose-proportional increases in maximum plasma concentration and area under the plasma concentration-time curve (AUC), with steady-state concentrations achieved after 4 weeks at the recommended dosage. Apalutamide has a mean oral bioavailability of approximately 100%, with a median time to reach maximum plasma concentration (tmax) of 2 hours. The drug and its active metabolite (N-desmethyl apalutamide) are highly bound to plasma proteins, with a mean apparent volume of distribution of 276 L at steady state. Apalutamide is mainly eliminated by metabolism, primarily through CYP2C8 and CYP3A4, with the active metabolite representing a significant portion of the total AUC following oral administration. The drug and its active metabolite are inducers of CYP3A4 and CYP2B6, moderate inhibitors of CYP2B6 and CYP2C8, and weak inhibitors of CYP2C9, CYP2C19, and CYP3A4. When co-administered with medications metabolized by these enzymes or transporters, Apalutamide may affect their pharmacokinetics. Age, race, and mild to moderate renal or hepatic impairment do not have clinically relevant effects on apalutamide's pharmacokinetic properties. However, the effects of severe renal impairment, end-stage renal disease, or severe hepatic impairment on Apalutamide's pharmacokinetics are not well understood. 2
Adverse Events
Adverse events (AEs) associated with apalutamide, have been extensively studied in clinical trials. In the phase III SPARTAN trial, which included 803 patients in the apalutamide group and 398 patients in the placebo group, AEs were reported by a high percentage of patients in both groups. The most commonly reported AE in both non-metastatic castration-resistant prostate cancer (nmCRPC) and metastatic CRPC (mCRPC) patients was fatigue. AEs led to discontinuation of the trial regimen in a notable percentage of patients in the Apalutamide group. Specifically, AEs leading to dose interruption or reduction of apalutamide were reported in 33% of patients, with grade 3 or 4 AEs occurring in 45.1% of patients. Serious AEs occurred in 24.8% of patients, and death due to an AE was reported in 1.2% of patients in the apalutamide group. Common AEs, occurring in over 15% of patients, included fatigue, hypertension, rash, diarrhea, nausea, weight loss, arthralgia, and falls. Other AEs of interest, occurring in less than 15% of patients, included fractures, dizziness, hypothyroidism, mental impairment disorder, seizures, pruritus, ischaemic heart disease, and heart failure. Furthermore, treatment-related AEs in the Apalutamide group included fatigue, rash, falls, fractures, hypothyroidism, and seizures. Grade 3 or 4 treatment-related AEs reported in the apalutamide group were predominantly rash, fractures, falls, and fatigue. It is important for healthcare providers to evaluate patients for fall and fracture risk and to initiate appropriate treatments for bone health and hypothyroidism when necessary. Additionally, permanent discontinuation of Apalutamide is recommended in patients who develop a seizure during treatment. These findings highlight the importance of closely monitoring patients for AEs while receiving Apalutamide treatment. 3
Reference
1. Clegg NJ, Wongvipat J, Joseph JD, et al ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–1503.
2. US FDA. ErleadaTM (apalutamide): prescribing information. 2018.
3. Al-Salama ZT. Apalutamide: First Global Approval. Drugs. 2018;78(6):699-705.
- Related articles
- Related Qustion
- What is Apalutamide Dec 26, 2023
Apalutamide is a next-generation oral androgen receptor (AR) inhibitor that Janssen is developing for the treatment of prostate cancer.
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Dec 16,2024Biochemical EngineeringOrnidazole can cause severe liver damage and fixed drug reactions, necessitating prompt recognition and withdrawal for appropriate management.....
Feb 6,2024APIApalutamide
956104-40-8You may like
- *Apalutamide
-

- $0.00 / 1g
- 2025-04-15
- CAS:956104-40-8
- Min. Order: 1g
- Purity: 0.99
- Supply Ability: 6kg
- Apalutamide
-

- $0.00 / 25Kg/Bag
- 2025-04-15
- CAS:956104-40-8
- Min. Order: 2Kg/Bag
- Purity: 0.99
- Supply Ability: 20 tons
- Apalutamide
-

- $0.00 / 25Kg/Bag
- 2025-04-15
- CAS:956104-40-8
- Min. Order: 2Kg/Bag
- Purity: 0.99
- Supply Ability: 20 tons