BIBW2992 DiMaleate: pharmacokinetic, clinical applications and safety
Nov 20,2023
General Description
BIBW2992 DiMaleate is a medication used for the treatment of certain types of lung cancer. A study conducted on healthy Chinese subjects demonstrated the bioequivalence of the test and reference formulations of BIBW2992 DiMaleate. The study found that both formulations were well tolerated, with no serious adverse events reported. BIBW2992 DiMaleate is a potent and selective irreversible tyrosine kinase inhibitor (TKI) that targets epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and HER4. It has shown efficacy in treating non-small cell lung cancer (NSCLC) and advanced HER2-positive breast cancer. However, it is important to note that the safety of BIBW2992 DiMaleate depends on individual patient characteristics and proper medical supervision. Common side effects include diarrhea, rash, dry skin, and nail changes, while more severe adverse effects may occur. Therefore, careful assessment and monitoring are necessary when prescribing this medication.

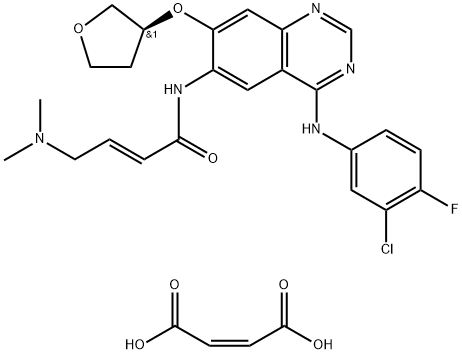
Figure 1. BIBW2992 DiMaleate
Pharmacokinetic
BIBW2992 DiMaleate is a medication used to treat certain types of cancer. In a study, researchers aimed to determine the bioequivalence of the test (T) and reference (R) formulations of BIBW2992 DiMaleate tablets in healthy Chinese subjects under fasted conditions. A study followed a randomized, open-label, crossover design with two periods and single-dose administration. A total of 60 healthy subjects were included and randomly assigned to either the T/R or R/T groups. Each subject received a single oral dose of either the test or reference formulation, with a 14-day washout period between doses. The pharmacokinetic parameters assessed for bioequivalence included maximum concentration (Cmax), area under the concentration-time curve (AUC) from time 0 to the last measurable concentration, and AUC from time 0 to infinity. The plasma concentrations of BIBW2992 DiMaleate were analyzed using liquid chromatography–tandem mass spectrometry. Adverse events were monitored through patient interviews and physical examinations to assess the safety of the formulations. Four subjects withdrew before the second dosing period. The 90% confidence intervals of the geometric mean ratios for Cmax, AUC from time 0 to the last measurable concentration, and AUC from time 0 to infinity were within the bioequivalence range of 80.0% to 125.0%. Based on these results, the study concluded that the test formulation of BIBW2992 DiMaleate was bioequivalent to the reference formulation in healthy Chinese subjects under fasted conditions. Both formulations were well tolerated, and no serious adverse events were observed during the study. In summary, this study provides evidence supporting the bioequivalence of the test and reference formulations of BIBW2992 DiMaleate in healthy Chinese subjects. 1
Clinical applications
BIBW2992 DiMaleate is a potent and selective irreversible tyrosine kinase inhibitor (TKI) that has shown promising clinical applications in various cancer treatments. The primary mechanism of action of BIBW2992 DiMaleate is its ability to inhibit the activity of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and HER4. By irreversibly binding to these receptors, it effectively blocks the downstream signaling pathways responsible for cell growth and division. This targeted inhibition makes BIBW2992 DiMaleate particularly effective in treating tumors that overexpress EGFR and HER2. Clinical studies have demonstrated the efficacy of BIBW2992 DiMaleate in several cancer types, including non-small cell lung cancer (NSCLC) and breast cancer. In NSCLC patients with EGFR mutations, BIBW2992 DiMaleate has shown superior progression-free survival compared to standard chemotherapy. It has also exhibited positive outcomes in advanced HER2-positive breast cancer patients who have progressed on trastuzumab-based therapy. In conclusion, BIBW2992 DiMaleate is a promising TKI with potent inhibitory effects on EGFR, HER2, and HER4. Its clinical applications have shown significant benefits in the treatment of NSCLC and advanced HER2-positive breast cancer. 2
Safety
BIBW2992 DiMaleate is a medication used for the treatment of certain types of lung cancer. When discussing its safety profile, it is important to consider multiple factors. Clinical trials have indicated that common side effects of BIBW2992 DiMaleate include diarrhea, rash, dry skin, and nail changes. Additionally, it may cause more severe adverse effects such as interstitial lung disease, hepatotoxicity, and gastrointestinal perforation. It is crucial to note that the safety of any medication depends on various factors, including individual patient characteristics, concurrent medications, and proper medical supervision. Therefore, before initiating treatment with BIBW2992 DiMaleate, it is essential to thoroughly assess the patient's medical history and closely monitor their response to the medication. 1
Reference
1. Shi P, Jiang X, Tao Y, Li T, Li X, Wang C, Liu Y, Ma Y, Gao X, Cao Y. Pharmacokinetic and Safety Comparison of 2 Afatinib Dimaleate Tablets in Healthy Chinese Volunteers Under Fasted Conditions: A Randomized, Open-Label, 2-Period, Single-Dose Crossover Study. Clin Pharmacol Drug Dev. 2022 Oct;11(10):1177-1183.
2. Arrieta O, Barrón F, Padilla MS, Avilés-Salas A, Ramírez-Tirado LA, Arguelles Jiménez MJ, Vergara E, Zatarain-Barrón ZL, Hernández-Pedro N, Cardona AF, Cruz-Rico G, Barrios-Bernal P, Yamamoto Ramos M, Rosell R. Effect of Metformin Plus Tyrosine Kinase Inhibitors Compared With Tyrosine Kinase Inhibitors Alone in Patients With Epidermal Growth Factor Receptor-Mutated Lung Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019 Nov 1;5(11):e192553.
- Related articles
- Related Qustion
- Side effects of afatinib dimaleate Mar 25, 2025
Afatinib dimaleate, the dimaleate form of afatinib, is an orally bioavailable anilinoquinazoline derivative that is an inhibitor of the epidermal growth factor receptor (ErbB; EGFR) family of receptor tyrosine kinases (RTKs) with antitumor
- Afatinib dimaleate: Chemical properties, Indications, Mechanism of action and Side effects Sep 2, 2024
Afatinib dimaleate is very soluble in water and aqueous buffers with a pH of 1 to 6 (> 50 mg/ mL). The free base is strongly lipophilic (log P = 4.7) but is strongly pH dependent.
- BIBW2992 Dimaleate - Pharmacodynamics Dec 13, 2019
Afatinib dimaleate (Tovok; BIBW2992; Gilotrif) is a salt form of Afatinib. Afatinib is a second-generation, orally administered, irreversible inhibitor of the ErbB family of tyrosine kinases.
Piperazine citrate paralyzes worms by inhibiting GABA receptors, treating certain parasitic infections effectively and safely.....
Nov 20,2023APIAmisulpride is a dopamine antagonist that has been used as an oral atypical antipsychotic. This drug is also a new drug for the management of postoperative nausea and vomiting.....
Nov 20,2023APIAfatinib dimaleate
850140-73-7You may like
Afatinib dimaleate manufacturers
- Afatinib dimaleate
-

- $0.00 / 1g
- 2025-11-18
- CAS:850140-73-7
- Min. Order: 1g
- Purity: 99%min
- Supply Ability: 1000g
- Afatinib DIMELATE
-
- $2800.00 / 1kg
- 2025-11-18
- CAS:850140-73-7
- Min. Order: 0.1kg
- Purity: 99
- Supply Ability: 10
- Afatinib Dimaleate
-

- $33.00 / 5mg
- 2025-11-10
- CAS:850140-73-7
- Min. Order:
- Purity: 99.87%
- Supply Ability: 10g