Bisoprolol Fumarate: Clinical Pharmacology and Detection Method
Sep 23,2024
General Description
Bisoprolol Fumarate is a selective beta-1 adrenoceptor antagonist commonly used to manage hypertension and heart failure. It has approximately 80% bioavailability, with peak plasma concentrations reached within 2 to 4 hours. Its pharmacodynamic effects include negative chronotropic action, reducing heart rate and cardiac output while minimally affecting stroke volume. Furthermore, Bisoprolol Fumarate has been shown to have minimal effects on lipid profiles and may enhance the efficacy of thiazide diuretics in lowering blood pressure. High-Performance Liquid Chromatography is employed for assessing the stability and compatibility of Bisoprolol Fumarate in formulations, ensuring its effectiveness and safety.

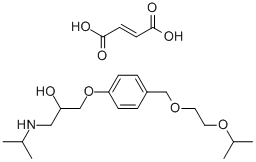
Figure 1. Bisoprolol fumarate
Clinical Pharmacology
Bisoprolol Fumarate is primarily recognized as a selective beta-1 adrenoceptor antagonist, widely used in the management of cardiovascular conditions such as hypertension and heart failure. This agent exhibits cardioselectivity, particularly at standard therapeutic doses. However, at higher doses of Bisoprolol Fumarate (≥ 20 mg), it can also inhibit beta-2 adrenoceptors, which are mainly present in bronchial and vascular smooth muscle. This characteristic necessitates the careful administration of the lowest effective dose of Bisoprolol Fumarate to preserve its selective action and mitigate potential side effects, especially in patients with respiratory concerns. 1
Pharmacokinetics and Metabolism
The pharmacokinetic profile of Bisoprolol Fumarate indicates that it has an absolute bioavailability of approximately 80% following oral administration. Importantly, the absorption of Bisoprolol Fumarate is not influenced by food intake, highlighting its practical usability in various treatment settings. After a dose adjustment, peak plasma levels of Bisoprolol Fumarate are typically reached within 2 to 4 hours, with half-life estimates ranging from 9 to 12 hours. The elimination of Bisoprolol Fumarate occurs through both renal and non-renal pathways, demonstrating a balanced metabolic clearance. In individuals with impaired renal function or liver cirrhosis, the half-life can increase significantly, influencing the overall pharmacokinetic behavior of this drug. 1
Pharmacodynamics and Clinical Implications
The primary pharmacodynamic effect of Bisoprolol Fumarate is its ability to induce negative chronotropic effects, which results in lowered heart rate during both rest and exertion. This reduction in heart rate is accompanied by decreases in cardiac output, albeit with minimal changes in stroke volume. The antihypertensive mechanism of Bisoprolol Fumarate appears multifactorial, involving decreased cardiac output, inhibition of renin release, and reduced sympathetic nervous system activity. In clinical studies, Bisoprolol Fumarate therapy has shown effectiveness in lowering heart rate induced by exercise and pharmacological stimuli. Furthermore, it exhibits minimal negative effects on lipid profiles, which is crucial for patients with concurrent cardiovascular conditions. Studies have also confirmed the additive effects of combining Bisoprolol Fumarate with thiazide diuretics, enhancing the blood pressure-lowering efficacy for patients suffering from mild to moderate hypertension. 1
Detection Method
High-Performance Liquid Chromatography Method
High-Performance Liquid Chromatography (HPLC) serves as a reliable analytical technique for assessing the stability and compatibility of Bisoprolol Fumarate in various formulations. In a recent study, researchers investigated the compatibility of Bisoprolol Fumarate with several excipients, including ascorbic acid and citric acid, through isothermal stress testing at elevated temperatures. The newly developed HPLC method enabled the precise quantification of Bisoprolol Fumarate content in stressed samples. Results indicated significant degradation of Bisoprolol Fumarate when exposed to certain excipients, showcasing the method's efficacy in evaluating the stability of this compound under extreme conditions. Such validation is vital in pharmaceutical formulation development, ensuring that Bisoprolol Fumarate remains effective and safe. 2
Simultaneous Estimation in Pharmaceutical Formulations
In addition to compatibility testing, HPLC is utilized for the simultaneous estimation of Bisoprolol Fumarate and hydrochlorothiazide in tablet formulations. This specific HPLC method employs a combination of potassium dihydrogen phosphate buffer and acetonitrile as the mobile phase, delivering optimal separation on a specialized column. Detection occurs at 228 nm, where the method demonstrates a linear response for different concentrations of Bisoprolol Fumarate. The developed HPLC method is not only specific and stable-indicating but also adheres to international guidelines for analytical method validation. Stressed sample analysis confirmed a high resolution between Bisoprolol Fumarate and its degradation products, ensuring accurate results. This approach is essential for both quality control and stability studies of formulations containing Bisoprolol Fumarate, signifying its importance in the pharmaceutical industry. 3
Reference
1. BISOPROLOL FUMARATE tablet. DailyMed. 2022; NDC Code(s): 51407-645-01.
2. Joshi SJ, Karbhari PA, Bhoir SI, Bindu KS, Das C. RP-HPLC method for simultaneous estimation of bisoprolol fumarate and hydrochlorothiazide in tablet formulation. J Pharm Biomed Anal. 2010; 52(3): 362-371.
3. Marothu VK, Yerramothu P, Gorrepati M, Majeti S, Mamidala SK, Nellutla A. Application of HPLC to assess the compatibility of bisoprolol fumarate with selected excipients in mixtures by isothermal stress testing. Ann Pharm Fr. 2015; 73(6): 442-451.
- Related articles
- Related Qustion
- Bisoprolol fumarate: Indications, Mechanism of Action and Side Effects Sep 9, 2024
Bisoprolol fumarate is the active ingredient in the prescription drug Bisoprolol, which belongs to the class of drugs known as beta-blockers.
- Synthesis and Detection method of Bisoprolol fumarate Aug 29, 2022
Bisoprolol fumarate is used to treat conditions such as hypertension and angina pectoris.
Fusidine, a topical antibiotic with a steroid-like structure, effectively treats skin infections like impetigo, with low side effects and high efficacy against resistant bacteria.....
Sep 23,2024APIBenzyldodecyldimethylammonium bromide is a versatile disinfectant effective against microorganisms, used in healthcare, and agriculture, but requires careful handling due to high toxicity.....
Sep 23,2024APIBisoprolol fumarate
104344-23-2You may like
Bisoprolol fumarate manufacturers
- Bisoprolol fumarate
-

- $0.00 / 1KG
- 2024-10-14
- CAS:104344-23-2
- Min. Order: 1KG
- Purity: 99.0%~101.0%; EP10.0
- Supply Ability: 1500kg/month
- Bisoprolol(Fumarate)
-
- $0.00 / 10mg
- 2024-09-20
- CAS:104344-23-2
- Min. Order: 10mg
- Purity: 98%
- Supply Ability: 500mg
- Bisoprolol fumarate
-

- $596.00 / 1KG
- 2024-08-30
- CAS:104344-23-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 1T