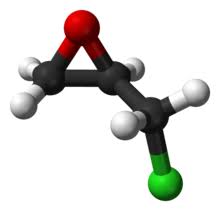
Butylchlorodihydroxytin - Reaction / Application on synthetic works
Nov 8,2019
The synonyms of butylchlorodihydroxytin are butyltin(IV) chloride dihydroxide, butyldihydroxidetin(IV) chloride, butyltin(IV) chloridedihydroxide, n-butylchlorotin (IV) dihydroxide, n-butyltin chloride dihydroxide, butyltin chloride dihydroxide, and n-butyltin dihydroxy chloride
Butylchlorodihydroxytin is an important Tin-based catalyst to synthetize polymers and organotin substituted products. [1-4].
Example 1
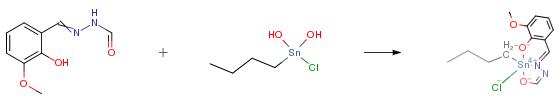
The product was prepared by refluxing N′-(2-hydroxy-3-methoxybenzylidene) formohydrazide 0.58 g (3.0 mmol) with dioctyltin (IV) oxide 1.09 g (3.0 mmol) in 100 mL dry toluene in 1:1 ratio. The water formed during the reaction was removed by Dean–Stark apparatus. The yellow solution obtained was rotary evaporated under reduced pressure and the solid formed was recrystallized from chloroform and n-hexane (4:1) mixture
Example 2

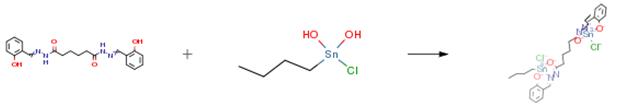
The product was prepared by refluxing N1′,N6′-bis(5-bromo-2-hydroxybenzylidene) adipodihydrazide 0.81g (1.5mmol) and dioctyltin (IV) oxide 1.09g (3.0mmol) in 100mL dry toluene in 1:2 ratio. The water formed during the reaction was removed by Dean-Stark apparatus. The yellow solution obtained was rotary evaporated under reduced pressure. A viscous liquid product was obtained.
Example 3
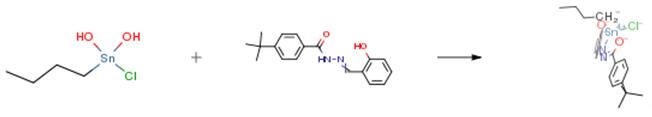
The product was prepared byrefluxing N1′,N6′-bis(2-hydroxybenzylidene) adipodihydrazide( 0.57 g, 1.5 mmol) with di-n-octyltin (IV) oxide(1.09 g, 3.0 mmol) in 100 mL dry toluene in 1:2 ratio. The water formed during the reaction was removed by Dean–Stark apparatus. The yellow solution obtained was rotary evaporated under reduced pressure viscous liquid product that was obtained. Yield: 72 percent. The product can also be prepared in the same way, using following precursors quantities: N1′,N6′-bis(2-hydroxybenzylidene) adipodihydrazide (0.57 g, 1.5 mmol) andbutyldihydroxidetin(IV) chloride (0.73 g, 3.0 mmol) werereacted in a 1:2 ratio. Solid product was recrystallized froma chloroform and n-hexane (4:1) mixture. Yield 75percent. mp 130–132 °C.
Example 4
References
1.Shujah S, Zia-Ur-Rehman, Muhammad N, Shah A, Ali S, Khalid N, Meetsma A. Bioactive hepta- and penta-coordinated supramolecular diorganotin(IV) Schiff bases[J]. Journal of Organometallic Chemistry, 2013, 741-742(1):59 - 66.
2. Shujah S, Zia-Ur-Rehman, Muhammad N, Shah A, Ali S, Meetsma A, Hussain Z. Homobimetallic organotin(IV) complexes with hexadentate Schiff base: Synthesis, crystal structure and antimicrobial studies[J]. Journal of Organometallic Chemistry, 2014, 759:19 - 26.
3. Shujah S, Ali S, Khalid N, Meetsma A. Supramolecular homobimetallic bis-diorganotin(IV) complexes of ditopic oxygen nitrogen donor ligand: synthesis, spectroscopic characterization, crystal structure and biological screening[J]. Journal of the Iranian Chemical Society, 2017, 14(12):2567 - 2578.
4. Shujah S, Ali S, Khalid N, Alam MJ, Ahmad S, Meetsma A. Synthesis, spectroscopic characterization, X-ray structure, DFT calculations, and antimicrobial studies of diorganotin (IV) complexes of monotopic oxygen nitrogen donor Schiff base[J]. Chemical Papers, 2018, 72(4):903 - 919.
- Related articles
- Related Qustion
- Butylchlorodihydroxytin: properties, applications and safety Dec 1, 2023
Butylchlorodihydroxytin has valuable applications but requires adherence to safety guidelines due to potential hazards.
Acarbose is a prescription medication. It comes as an oral tablet.Acarbose is available as the brand-name drug Precose. This drug may be used as part of a combination therapy.....
Nov 7,2019APIEpichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organic solvents.....
Nov 8,2019Organic ChemistryButylchlorodihydroxytin
13355-96-9You may like
Butylchlorodihydroxytin manufacturers
- Butylchlorodihydroxytin
-

- $18.60 / 1KG
- 2024-10-11
- CAS:13355-96-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5000kg
- Butylchlorodihydroxytin
-

- $1.00 / 1KG
- 2020-01-13
- CAS:13355-96-9
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100KG
- Butyltin CAT (Butylchlorodihydroxytin; Butyltin chloride dihydroxide)
-

- $200.00 / 1mkg
- 2019-05-22
- CAS:13355-96-9
- Min. Order: 10g
- Purity: 99.9%
- Supply Ability: 1000kg