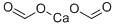Calcium Formate: An In-Depth Analysis for Chemical Professionals
Nov 1,2024
Introduction
Calcium formate is a highly soluble calcium salt containing 30.8% calcium by weight (compared with 40.0% for calcium carbonate and 24.1% for calcium citrate). It is an odourless, free-flowing white crystalline solid. Calcium formate was introduced as a hydration accelerator for cement during the 1970s and is predominately used to offset the retarding effect of water-reducing admixtures. Calcium formate has a low solubility of around 160 g/l at 20 °C, and it is usually added as a powder, which might create dispersing problems.

Chemical reactions
Calcium formate can be used to prepare solutions of other water-soluble formate salts. For example, the addition of nickel (II) sulfate to a solution of calcium formate results in the precipitation of insoluble calcium sulfate, leaving nickel (II) formate in solution:
Ca(OOCH)2 + NiSO1 → CaSO4 + Ni(OOCH)2
In a similar reaction, sulfuric acid reacts with calcium formate to provide low-cost solutions of formic acid:
Ca(OOCH)2 + H2 SO4 → CaSO4 + 2HCOOH
When heated, calcium formate decomposes to calcium carbonate and formaldehyde:
Ca(OOCH)2 → CaCO3 + HCHO
Continued heating at higher temperatures results in the breakdown of these decomposition products:
HCHO → CO + H2
CaCO3 → CaO + CO2
Calcium formate can be a low-cost reducing agent in converting carboxylic acids to aldehydes. This process is an effective substitute for the troublesome Rosenmund reduction.
(RCOO)2Ca + (HCOO)2CA → 2RCH + 2CaCO3
An efficient source of calcium for dietary supplementation
Calcium is an essential nutrient required in substantial amounts, but many diets are deficient in calcium, making supplementation necessary or desirable. Calcium formate is a convenient source of calcium and formate ions for aqueous solutions. In addition, calcium formate's high solubility and calcium content suggest that it might be an efficient source of calcium for dietary supplementation. However, no information is available concerning the bioavailability of calcium from calcium formate.
Fisher et al. compare the oral bioavailability of calcium from calcium formate, a new experimental dietary calcium supplement, to calcium citrate and calcium carbonate. In a four-way crossover study, either a placebo or 1200 mg of calcium as calcium carbonate, calcium citrate, or calcium formate were administered orally to 14 healthy adult female volunteers who had fasted overnight. After calcium carbonate, the maximum rise in serum calcium (~4%) and the fall in serum intact parathyroid hormone 1– 84 (iPTH) (~20 – 40%) did not differ significantly from placebo. After calcium citrate, the changes were modestly but significantly (p <0.05) greater, but only at 135 to 270 min after ingestion. In contrast, within 60 min after calcium formate, serum calcium rose by approximately 15%, and serum iPTH fell by 70%. The mean increment in the area under the plasma concentration-time curve (0 –270 min) for serum calcium after calcium formate (378 mg·min/dl) was double that for calcium citrate (178 mg·min/dl; p<0.01), whereas the latter was only modestly greater than either placebo (107; p<0.05) or calcium carbonate (91; p<0.05). In this study, calcium formate was clearly superior to both calcium carbonate and calcium citrate in its ability to deliver calcium to the bloodstream after oral administration. Calcium formate may offer significant advantages as a dietary calcium supplement.
References:
[1] ROBERT P HANZLIK D H F Stephen C Fowler. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate.[J]. Journal of Pharmacology and Experimental Therapeutics, 2005, 313 3. DOI:10.1124/jpet.104.081893.
[2] HEIKAL M. Effect of calcium formate as an accelerator on the physicochemical and mechanical properties of pozzolanic cement pastes[J]. Cement and Concrete Research, 2004, 34 6: 907-1072. DOI:10.1016/j.cemconres.2003.11.015.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTrenbolone Cyclohexylmethylcarbonate sold under the brand names Parabolan and Hexabolan, is a synthetic, injected anabolic–androgenic steroid (AAS) of the nandrolone group and an androgen ester – specifically.....
Oct 23,2024APICalcium formate
544-17-2You may like
- Calcium formate
-

- $999.00/ kg
- 2025-12-16
- CAS:544-17-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Calcium formate
-
- 2025-12-15
- CAS:544-17-2
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Calcium formate
-

- $10.00 / 1KG
- 2025-12-11
- CAS:544-17-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt