Camphor, D-Camphor and L-Camphor
Nov 1,2023
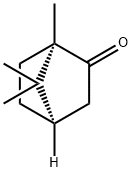
Camphor
Camphor is a central nervous system (CNS) stimulant with effects that range from mild CNS excitation to generalized seizures. Camphor is highly lipid soluble and easily crosses the blood–brain barrier. The natural form of camphor is D-camphor, while the synthetic version is a mixture of the D and L enantiomers. As an antiseptic, analgesic, antipruritic, and counterirritant agent, Camphor has a long history. It is also used as a decongestant and cough suppressant in the form of ointments, oils, and inhalants. Recently, investigations have shown that camphor-containing compounds have uterotrophic, antitussive, anticonvulsant, nicotinic receptor blocking, antiimplantation, antiestrogenic, as well as estrogenic activities, and reduced serum triglyceride and thyroid hormone.

D-Camphor
D-Camphor is a volatile terpenoid that can be used as a flavoring agent and a fragrance ingredient. D-camphor are crystalline monoterpene that can be isolated, by distillation, from Cinnamomum camphora(L.) J. Presl. It has been identified as the major volatile component in the essential oil extracted from the leaves of Blumea balsamifera and Ashe juniper. This compound is always added to eye drops as a fragrance component which also includes l-menthol, phenylethyl alcohol, and geraniol[1]. The structure of d-camphor is similar to that of borneol with sedative activity; they only differ in that the ketone group of d-camphor is substituted by a hydroxy group in borneol. Both d-camphor and borneol have potent sedative activities with optimal concentrations of 4×10–4 and 4×10–3 mg/cage, respectively.
L-Camphor
(S)-camphor, or L(-)-Camphor, is a stereoisomer of Camphor, a bicyclic monoterpene known to potentiate both heat and cold sensations. L-camphor is one of the main components of essential oil. L-camphor appears as colorless or white crystals. It has an aromatic and penetrating smell. The taste is cool and slightly bitter. (S)-camphor is not the naturally occurring stereoisomer but displays similar TRPV channel affinity and current inhibition[2].
Toxicity
Ingestion of 2 g of camphor is sufficient to produce toxic effects in adults. Initial symptoms may occur within 5 to 15 min after ingestion and include nausea and vomiting, oral and epigastric burning, a feeling of warmth, and a headache. These symptoms may progress to altered mental status, convulsions, coma, and death. Death usually is attributed to respiratory failure or neurologic complications[3]. Clinical toxicity typically resolves within 24 hours in survivors. Cardiovascular toxicities include tachycardia, prolonged QTc and QRS intervals, atrioventricular conduction block, and ST segment changes. Myocarditis and depressed ventricular systolic function may occur following camphor ingestion.
References
[1] Oshima T, et al. Sedative effects of l-menthol, d-camphor, phenylethyl alcohol, and geraniol. Journal of Natural Medicines, 2021; 75: 319–325.
[2] Choulitoudi E, et al. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packaging and Shelf Life, 2017; 12: 107-113.
[3] Klenkler R, et al. Chapter 3 - Environmental Toxins and the Heart. Heart and Toxins, 2015; 75-132.
- Related articles
- Related Qustion
- D-Camphor: Sources, Biosynthesis and Antimicrobial Activity May 9, 2024
D-Camphor, sourced from α-pinene and various trees, has weak antimicrobial activity but enhances other compounds' effects, vital for industrial use.
- D-camphor:a specific enantiomer of camphor Dec 20, 2023
D-Camphor: Versatile compound used in pharmaceuticals, fragrances, and plastics; handle with care due to potential toxicity and irritant properties.
Sunitinib is an oral multiple tyrosine kinase receptor inhibitor. It is usually well tolerated but can cause fatigue, malaise, and rash.....
Oct 31,2023Biochemical EngineeringO-Mesitylenesulfonylhydroxylamine is an oxidizing and aminating reagent with great potential to oxidatively eliminate the cysteine thiol modification to dehydroalanine.....
Nov 1,2023Organic reagentsD-CAMPHOR
464-49-3You may like
- Is o-xylene harmful to humans?
Jul 24, 2024
- The toxicity of Bifenthrin
Jul 23, 2024
- The uses of 3-Methyl-1-butanol
Jul 15, 2024
- D-CAMPHOR; Camphor tree extract
-

- $0.00 / 1KG
- 2024-07-23
- CAS:464-49-3
- Min. Order: 1KG
- Purity: ≥98% HPLC
- Supply Ability: 1000KG
- D-CAMPHOR
-

- $10.00/ kg
- 2024-07-22
- CAS:464-49-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 tons
- D-CAMPHOR
-

- $5.00 / 1KG
- 2024-07-10
- CAS:464-49-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20TONS