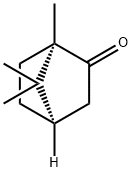
D-camphor:a specific enantiomer of camphor
Dec 20,2023
Introduction
"D-CAMPHOR" likely refers to D-camphor, a specific enantiomer of camphor. Camphor is a white, crystalline substance with a strong odor. It is commonly found in the wood of the camphor laurel tree (Cinnamomum camphora) and is also synthesized chemically. Camphor exists in two main forms, or enantiomers: D-camphor (dextrorotatory) and L-camphor (levorotatory). Enantiomers are mirror-image isomers of a molecule, and they have different optical activities. D-camphor rotates polarized light to the right, while L-camphor rotates it to the left. Camphor has been used for various purposes, including as a flavoring agent in food, in the production of pharmaceuticals, and in traditional medicine. It is also used in the manufacturing of some plastics, as well as in mothballs and other insect repellents.
Application
The dextrorotatory enantiomer of camphor, serves diverse applications across industries. In pharmaceuticals, it acts as a crucial chiral building block for the synthesis of certain drugs and intermediates. The flavor and fragrance industry employs D-Camphor to impart a minty, camphoraceous note to perfumes and scented products. Additionally, it finds utility in plastics and polymers, where it functions as a plasticizer. Commonly used in mothballs and insect repellents, D-Camphor exhibits insect-repelling properties. In traditional medicine, it has historical applications for its cooling and analgesic effects. Furthermore, D-Camphor plays a role in chemical research and synthesis, particularly in the preparation of chiral compounds and asymmetric syntheses, underscoring its versatility across various fields. However, it is crucial to adhere to safety guidelines and regulations to ensure proper handling and usage.
An enantiomer of nabscessin A (1), an aminocyclitol amide with antimicrobial activity, was synthesized from myoinositol and dimethyl D-camphor acetal in 14 steps. Formal synthesis of natural nabscessin A was also achieved through the new approach to access both enantiomers of 4,5-di-O-benzyl-myo-inositol, derived from the same set of starting materials. This synthesis features utilizations of the existing framework of myo-inositol and a regioselective esteripcation3.

Figure 1 Asymmetric Synthesis of Nabscessin A from Inositol and D‑Camphor
Synthesis
The synthesis of D-Camphor involves a multistep process starting with camphor oxide, obtained through the oxidation of camphor, either extracted from the camphor tree or synthesized chemically. The reduction of camphor oxide is then carried out, favoring the stereoselective formation of D-Camphor, often utilizing chiral catalysts to enhance specificity. Subsequent purification steps, such as distillation or recrystallization, refine the crude product. Analysis techniques, including chiral chromatography and polarimetry, assess optical purity and overall quality. The synthesis pathway underscores the versatility of D-Camphor, finding applications in pharmaceuticals, flavor and fragrance, plastics, insect repellents, traditional medicine, and chemical research. Adherence to safety guidelines and regulations is essential throughout the synthesis process.
Safety
Camphor is present in several over-the-counter compounds of questionable use and therefore may be ingested by small children. Because seizures may follow ingestion of certain amounts, appropriate treatment is needed, including the use of anticonvulsants2. The safety of D-Camphor depends on its proper handling, use, and exposure precautions. Here are some considerations: Toxicity: D-Camphor is generally considered safe when used in appropriate amounts. However, excessive exposure, ingestion, or inhalation can lead to toxicity symptoms, including nausea, vomiting, headache, and dizziness. Skin Sensitization: Direct contact with D-Camphor can cause skin irritation or sensitization in some individuals. It's important to use appropriate personal protective equipment, such as gloves, when handling the compound. Inhalation: Inhalation of D-Camphor vapors in high concentrations can irritate the respiratory system. Ensure proper ventilation in areas where the compound is used, and use respiratory protection if needed. Ingestion: D-Camphor should not be ingested, as it can be toxic. Keep it out of reach of children and ensure that it is stored in a secure and labeled container. Eye Contact: Avoid eye contact with D-Camphor, as it can cause irritation. In case of accidental exposure, rinse eyes thoroughly with water. Fire Hazard: D-Camphor is combustible, and care should be taken to avoid open flames or sparks in its vicinity. Store it away from heat sources and oxidizing agents. Environmental Impact: Dispose of D-Camphor waste according to local regulations. It is essential to consider the environmental impact of its disposal and follow proper waste disposal procedures.One report a case of hepatotoxicity in a 2-month-old baby after a camphor-containing cold remedy was applied dermally. Liver function tests returned to normal after the application of the cold remedy was discontinued. Ingestion of camphor can cause severe liver and central nervous system injury, and neurotoxicity has been observed after exposure to camphor through the skin. Hepatotoxicity after dermal application of camphor has never been reported1.
Reference
1. Uc A, Bishop WP, Sanders KD. Camphor hepatotoxicity. South Med J. 2000 Jun;93(6):596-8.
2. Siegel E, Wason S. Camphor toxicity. Pediatr Clin North Am. 1986 Apr;33(2):375-9.
3. Wu HY, Wang WY, Feng CC, Hou DR. Asymmetric Synthesis of Nabscessin A from Inositol and d-Camphor. J Org Chem. 2020 Oct 16;85(20):13153-13159.
- Related articles
- Related Qustion
- D-Camphor: Sources, Biosynthesis and Antimicrobial Activity Nov 1, 2024
D-Camphor is a colorless or white crystals. Sublimes. Flash point 149°F. It burns readily with a bright, smoky flame.
- Camphor, D-Camphor and L-Camphor Nov 1, 2023
Camphor is highly lipid soluble and easily crosses the blood–brain barrier. The natural form of camphor is D-camphor, while the synthetic version is a mixture of the D and L enantiomers.
Propylparaben, a paraben compound, is widely used for its antimicrobial and preservative properties in various consumer products. The majority of it is excreted as non-specific metabolites.....
Dec 20,2023APIThe formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the SCl2 molecule.....
Dec 20,2023Inorganic chemistryD-CAMPHOR
464-49-3You may like
- Natural Camphor
-

- $0.00 / 1KG
- 2025-04-25
- CAS:464-49-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500kg/month
- camphor
-

- $0.00/ kg
- 2025-04-25
- CAS:464-49-3
- Min. Order: 25kg
- Purity: 96.00%
- Supply Ability: 1000kg
- D-CAMPHOR
-

- $6.00 / 1kg
- 2025-04-25
- CAS:464-49-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 2000KG/Month