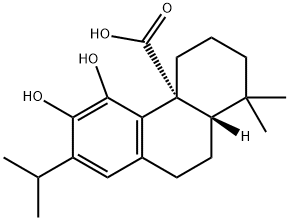
Carnosic acid: an antioxidantant and antimicrobial compound
Mar 11,2025
Introduction
Carnosic acid is a phenolic (catecholic) diterpene, endowed with antioxidative and antimicrobial properties. It is increasingly used within food, nutritional health and cosmetics industries. Carnosic acid was first discovered by Linde in Salvia officinalis L. Later, Wenkert et al. (1965) found carnosic acid at much higher levels (∼3% on weight basis of air-dried leaves) in Rosmarinus officinalis L. leaves. Since then, rosemary cultivars highly abundant in carnosic acid (4–10% on weight basis of air-dried leaves) were developed, including VAU3, Daregal, Farinole, 4 English, Severn Seas, Miss Jessops Upright, 1 English, Lighthorne culinary. The molecule has also been found in other Salvia species and other genera of the Lamiaceae (
Luis, 1991). Apart from the influence of the genetic background, contents in carnosic acid may also be modulated by growth conditions
. Recently, Tounekti and Munné-Bosch (2012) have reviewed certain aspects of phenolic diterpene biology, with a particular focus on the physiological, rather than trans-genetic, approaches, to enhancing and improving the phenolic diterpene levels and composition in Salvia and Rosmarinus plants and plant extracts. For instance, authors reported that the English climate favours the production of carnosic acid more than the warmer, more arid environmental conditions found in Mediterranean countries where rosemary and sage are typically found.

Furthermore, rosemary plants subjected to enhanced levels of UV-B radiation display higher yields of carnosic acid than non-treated plants. Moreover, water, salinity, intense light, and heat stress seem to negatively affect carnosic acid concentrations. Although stress conditions alone seem to decrease levels in carnosic acid, when applied together with supplements, they result in high yields in phenolic diterpenes. This was confirmed when low amounts of fertilizer or kinetin were supplemented to plants upon saline stress. Carnosic acid is a phenolic diterpene with a formula C20H28O4. It belongs to the largest class of over 50,000 plant secondary metabolites termed terpenoids, also known as isoprenoids or terpenes. Because carnosic acid contains a phenolic group, it is often classified among polyphenols. Yet, its cellular distribution, biosynthetic pathway, solubility properties and roles substantially differ from the majority of polyphenolic classes and rather resemble terpenoids such as tocopherols and carotenoids.[1]
Biosynthesis of carnosic acid
The biosynthesis of carnosic acid has not entirely been unravelled. Investigations have mainly focused on general terpenoid biosynthesis, with specific studies regarding diterpenes in Salvia, whereas rosemary has rarely received any attention. Carnosic acid biosynthesis probably follows the ‘biogenetic isoprene rule’ and it can be reasonably presumed that the construction of carnosic acid would involve enzyme-assisted electrophilic elongation, cyclisation and rearrangements of the basic skeleton. Carnosic acid belongs to the labdane-related class of diterpenoids, whose biosynthesis is most probably initiated by a sequential pair of cyclisation reactions. Due to the plastidial localisation of diterpene synthases, plant diterpenoids normally originate from the plastidic 1-deoxyxylulose-5-phosphate (DXP) pathway. However, a contribution of the cytosolic mevalonate (MVA) pathway is not to be excluded, given the significant number of studies showing the existence of cross-talk between the two pathways . Although no biosynthesis pathway for carnosic acid of rosemary has yet been proposed, Brückner et al. (2014) suggest that one reasonable intermediate is abietatriene, tricyclic abietane diterpene with an aromatized C-ring and molecular mass of 270. Indeed, the typical catechol group would be produced by double hydroxylation of abietatriene on the C-ring. Multiple oxidations on the 20-carbon backbone of the diterpenoid would provide the carboxyl group present in carnosic acid. Brückner et al. (2014) also suggest that since the cyclisation of GGDP by olefin-producing diterpene synthases typically results in products with a parent mass of 272 rather than 270, it seems unreasonable to think that abietatriene would be a direct product of such enzymes. Authors suggest that biosynthetic pathway en route to carnosic acid would involve the transformation of GGDP into copalyl diphosphate (CDP), which would convert into miltiradiene. Miltiradiene contains a cyclohexa-1,4-diene moiety that imposes a planar configuration on the distal ring, which is ideally placed for aromatisation, as required for the production of carnosic acid.[2]
Carnosic acid is also an antimicrobial compound
Rosemary extracts rich in carnosic acid have been shown to have antimicrobial activity and studies on various pathogens have been performed to determine which rosemary compounds were responsible for this activity.[3]The impact of carnosic acid was assessed by evaluating the antilisterial effect of two rosemary extracts containing 20 or 40% carnosic acid. Both showed an antimicrobial activity but the minimal inhibitory concentration (MIC) value of the later was two times lower than the former, suggesting that carnosic acid was the most active compound. This result was confirmed by determining the antimicrobial activity of 8 major rosemary phenolics. Carnosic acid was thus shown to have the strongest antilisterial activity when tested for 24 h at 30 °C after inoculation. This antilisterial activity was further improved at low pH and high NaCl content. Other Gram positive bacteria, such as Bacillus, Enterococcus, Streptococcus or Staphylococcus, or Gram negative bacteria, such as Escherichia, Salmonella or Campylobacter, were also responsive to carnosic acid. Even if most studies showed only a moderate effect on Gram positive and an even less intense effect on Gram negative bacteria, synergisms may occur with other antimicrobials.
It was shown for instance on Tetracycline, Erythromycin or Ethidium Bromide which antimicrobial actions were improved up to 8-fold against Staphylococcus aureus by addition of 10 μg/mL carnosic acid and up to 16-fold against Enterococcus faecium and Enterococcus faecalis by addition of 8 μg/mL carnosic acid. Mechanisms involved in antimicrobial activities of phenolic diterpenes have not yet been elucidated, but it has been suggested that the lipophilic structure of these compounds allows them to insert into the bacterial membrane (Cowan, 1999), where their hydrogen-bond-donor group(s) could interact with membrane phosphorylated groups (
Souza et al., 2011) or that carnosic acid acts as a modulator of the ethidium bromide efflux which is responsible for membrane permeability.
Evidence that carnosic acid is an antioxidant
Carnosic acid plus carnosol have been suggested to account for over 90% of the antioxidant properties of rosemary extract, although this has not yet been systematically verified. This high contribution of carnosic acid to antioxidative response of rosemary extract is probably also attributed to the great abundance of carnosic acid as compared to other rosemary phenolic diterpenes. It has been proposed that the radical scavenging activity of carnosic acid follows a mechanism analogous to that of other antioxidants, including α-tocopherol, and is caused by the presence of the two O-phenolic hydroxyl groups found at C11 and C12 (catechol moiety). Upon oxidation, carnosic acid and α-tocopherol display different antioxidative capacities that depend upon the lipid composition of the matrix and more so upon oxidative conditions. In emulsions, at 37 °C α-tocopherol better preserves lipids from oxidation than carnosic acid. At higher temperatures (60 °C), α-tocopherol is not as efficient as carnosic acid in protecting lipids from oxidation (Huang et al., 1996). Yet at higher temperatures (60 °C) carnosic acid is consumed faster than α-tocopherol, indicating that it is the oxidation products of carnosic acid that contribute to the greater antioxidative response. Moreover, it is methyl carnosoate, rather than carnosic acid or α-tocopherol, that is the most active antioxidant in w/o emulsions but it is less active than Trolox in o/w emulsions.
References
[1] Abreu, M. E., Müller, M., Alegre, L., & Munné-Bosch, S. (2008). Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. Journal of the Science of Food and Agriculture, 88(8), 2648-2653.
[2] Achour, S., Khelifi, E., Attia, Y., Ferjani, E., & Hellal, A. N. (2012). Concentration of antioxidant polyphenols from Thymus capitatus extracts by membrane process technology. Journal of Food Science, 77(4), 703-709.
[3] Aoyagi, Y., Takahashi, Y., Satake, Y., Takeya, K., Aiyama, R., Matsuzaki, T., Hashimoto, S., & Kurihara, T. (2006). Cytotoxicity of abietane diterpenoids from Perovskia abrotanoides and of their semisynthetic analogues. Bioorganic & Medicinal Chemistry, 14(15), 5285-5291.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering(S)-(+)-3-hydroxytetrahydrofuran, a key intermediate for the synthesis of afatinib, empagliflozin, fosamprenavir, amprenavir and other chemotherapy agents.....
Mar 11,2025Pharmaceutical intermediatesCarnosic acid
3650-09-7You may like
- Carnosic acid
-

- $10.00 / 1ASSAYS
- 2025-12-20
- CAS:3650-09-7
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 1 ton
- Carnosic acid
-

- $0.00 / 1kg
- 2025-12-19
- CAS:3650-09-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500
- Carnosic acid
-

- $0.00 / 1kg
- 2025-12-19
- CAS:3650-09-7
- Min. Order: 1kg
- Purity: 5-10%
- Supply Ability: 1000kg