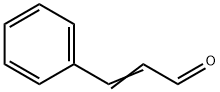
Cinnamaldehyde: Biosynthesis, applications, derivatives and metabolism
May 18,2023
General description
Cinnamaldehyde is an organic compound which occurs naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway.[1] This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. The essential oil of cinnamon bark is about 90% cinnamaldehyde. Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is trans-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. As such, the molecule can be viewed as a derivative of acrolein. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towards the visible. Its appearance is as follows:

Figure 1 Appearance of Cinnamaldehyde.
Biosynthesis
Biosynthesis of cinnamaldehyde begins with deamination of L-phenylalanine into cinnamic acid by the action of phenylalanine ammonia lyase (PAL).[2] PAL catalyzes this reaction by a non-oxidative deamination. This deamination relies on the MIO prosthetic group of PAL.[3] PAL gives rise to trans-cinnamic acid. In the second step, 4-coumarate–CoA ligase (4CL) converts cinnamic acid to cinnamoyl-CoA by an acid–thiol ligation. 4CL uses ATP to catalyze the formation of cinnamoyl-CoA. 4CL effects this reaction in two steps. 4CL forms a hydroxycinnamate–AMP anhydride, followed by a nucleophile attack on the carbonyl of the acyl adenylate. Cinnamoyl-CoA is reduced by NADPH catalyzed by CCR (cinnamoyl-CoA reductase) to form cinnamaldehyde.
Applications
The most obvious application for cinnamaldehyde is as flavoring in chewing gum, ice cream, candy, eliquid and beverages; use levels range from 9 to 4,900 parts per million (ppm) (that is, less than 0.5%). It is also used in some perfumes of natural, sweet, or fruity scents. Almond, apricot, butterscotch, and other aromas may partially employ the compound for their pleasant smells. Cinnamaldehyde can be used as a food adulterant; powdered beechnut husk aromatized with cinnamaldehyde can be marketed as powdered cinnamon. Some breakfast cereals contain as much as 187 ppm cinnamaldehyde.[4] Cinnamaldehyde has been tested as a safe and effective insecticide against mosquito larvae. A concentration of 29 ppm of cinnamaldehyde kills half of Aedes aegypti mosquito larvae in 24 hours. Trans-cinnamaldehyde works as a potent fumigant and practical repellant for adult mosquitos.
Cinnamaldehyde is also known as a corrosion inhibitor for steel and other ferrous alloys in corrosive fluids such as hydrochloric acid. It is believed that this is achieved by polymerization to form a protective film on the metal surface.[5] It can be used in combination with additional components such as dispersing agents, solvents and other surfactants. Cinnamaldehyde is also a potent inducer of apoptosis via ROS-mediated mitochondrial permeability transition in human promyelocytic leukemia HL-60 cells. Some early evidence shows that cinnamaldehyde blocks formation of Tau protein aggregation into neurofibrillary tangles, a major pathology in Alzheimer's Disease. Cinnamaldehyde also has antimicrobial properties. It is believed that the antimicrobial properties arise from the aldehyde group on Cinnamaldehyde.
Derivatives
Numerous derivatives of cinnamaldehyde are commercially useful. Dihydrocinnamyl alcohol occurs naturally but is produced by double hydrogenation of cinnamaldehyde. It has the fragrances of hyacinth and lilac. Cinnamyl alcohol similarly occurs naturally and has the odor of lilac but can be also produced starting from cinnamaldehyde.[6] Dihydrocinnamaldehyde is produced by the selective hydrogenation of the alkene subunit. α-Amylcinnamaldehyde and α-hexylcinnamaldehyde are important commercial fragrances, but they are not prepared from cinnamaldehyde. Hydrogenation of cinnamaldehyde, if directed to the alkene, gives hydrocinnamaldehyde.
Metabolism
Cinnamaldehyde occurs widely, and closely related compounds give rise to lignin. All such compounds are biosynthesized starting from phenylalanine, which undergoes conversion.[15] Cinnamoyl-CoA reductase is an enzyme responsible for the production of cinnamoyl-CoA from cinnamaldehyde. Autoxidation produces cinnamic acid.
References
[1]Gutzeit, Herwig (2014). Plant Natural Products: Synthesis, Biological Functions and Practical Applications. Wiley. pp. 19–21.
[2]Koukol, J.; Conn, E. E. (1961-10-01). "The metabolism of aromatic compounds in higher plants. IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare". The Journal of Biological Chemistry. 236: 2692–2698.
[3]Kong, Jian-Qiang (2015-07-20). "Phenylalanine ammonia-lyase, a key component used for phenylpropanoids production by metabolic engineering". RSC Advances. 5 (77): 62587–62603.
[4]Friedman, M.; Kozuekue, N.; Harden, L. A. (2000). "Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry". Journal of Agricultural and Food Chemistry. 48 (11): 5702–5709.
[5]Growcock, F. B.; Frenier, W. W.; Andreozzi, P. A. (1989). "Inhibition of Steel Corrosion in HCl by Derivatives of Cinnamaldehyde". Corrosion. 45 (12): 1007–1015.
[6]Zucca, P.; Littarru, M.; Rescigno, A.; Sanjust, E. (2009). "Cofactor recycling for selective enzymatic biotransformation of cinnamaldehyde to cinnamyl alcohol". Bioscience, Biotechnology, and Biochemistry. 73 (5): 1224–1226.
- Related articles
- Related Qustion
- Pharmacological activities and medical applications of Cinnamaldehyde Dec 16, 2024
Cinnamaldehyde is a natural compound extracted from the plant cinnamon. It has tremendous therapeutic uses because of its antimicrobial, antidiabetic, anti-inflammatory and anticancer properties.
- The hypoglycemic, hypolipidemic, and Antitumorigenic effects of Cinnamaldehyde Nov 27, 2023
Cinnamaldehyde has been used as a natural flavoring and fragrance agent in kitchens and industry.
- Synthesis and Uses of Cinnamaldehyde Nov 28, 2019
Cinnamaldehyde is a pale yellow gelatinous liquid and an organic compound, which is responsible for the taste and smell of the cinnamon spice. It is also identified by various names such as 3- beta-phenylacrolein, (E)-Cinnamaldehyde and cin
Iodine is quite reactive, but it is much less reactive than the other halogens.....
May 18,2023Inorganic chemistryFurfurylamine (FLA) is a key furan-based compound for the production of food additives, fuel additives, polymers, fibers, perfumes, and pharmaceuticals.....
May 18,2023Amino Acids and DerivativesCinnamaldehyde
104-55-2You may like
- Cinnamaldehyde
-

- $5.00 / 200KG
- 2025-04-22
- CAS:104-55-2
- Min. Order: 1KG
- Purity: ≥98%
- Supply Ability: 200mt/year
- Cinnamaldehyde
-

- $0.00 / 180kg
- 2025-04-22
- CAS:104-55-2
- Min. Order: 1000kg
- Purity: 98.0%
- Supply Ability: 100ton/month
- Cinnamic aldehyde
-

- $100.00 / 100KG
- 2025-04-21
- CAS:104-55-2
- Min. Order: 1KG
- Purity: 99.9%
- Supply Ability: 20 tons