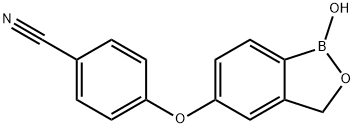
Crisaborole: A Boron-Containing Molecule with Therapeutic Efficacy in Treating Atopic Dermatitis
Feb 6,2024
General Description
Crisaborole is a boron-containing molecule that inhibits PDE4, leading to increased cAMP levels and inhibition of pro-inflammatory cytokines. It is well-tolerated when applied topically and does not cause QT interval prolongation. Systemic absorption is minimal, with rapid metabolism into inactive metabolites. Crisaborole has shown therapeutic efficacy in treating mild to moderate atopic dermatitis, as demonstrated by clinical trials. It improves disease severity, symptoms, and related endpoints such as erythema and itching. Overall, crisaborole ointment 2% effectively treats atopic dermatitis by reducing inflammation and providing relief from symptoms.

Figure 1. Crisaborole
Pharmacodynamic Properties
Crisaborole is a boron-containing, small molecule inhibitor of PDE4 with a molecular weight of 251.1 Da. Its low molecular weight allows it to effectively penetrate the skin and target cells. The presence of the boron atom enhances the binding capacity and selectivity of crisaborole for its target. It has been observed that replacing the boron atom results in a loss of PDE4 enzyme and cytokine inhibitory activity. The exact mechanism of action of crisaborole in treating atopic dermatitis is not fully understood. However, it is known to inhibit PDE4, leading to increased intracellular cAMP levels. This, in turn, inhibits the production of pro-inflammatory and T-cell cytokines. In human peripheral blood mononuclear cells, crisaborole has been shown to inhibit the production of tumor necrosis factor-a, interferon-c, IL-2, IL-5, and IL-10. In terms of tolerability, a phase I study on 32 healthy volunteers demonstrated that crisaborole ointment 2% applied twice daily for 21 days was well tolerated on sensitive areas such as the face, hairline, genitals, and intertriginous areas. Almost all tolerability assessments (99%) showed no signs or symptoms of irritation. Furthermore, therapeutic doses of crisaborole ointment 2% are not expected to cause any clinically relevant prolongation of corrected QT intervals. 1
Pharmacokinetic Properties
Crisaborole is minimally systemically absorbed following topical administration, with systemic exposure being rapid and increasing with the affected body surface area. In a clinical study on patients with mild to moderate atopic dermatitis, crisaborole ointment 2% showed a mean maximum plasma concentration of 127 ng/mL, median time to maximum concentration of 3 hours, and mean area under the concentration-time curve from time 0 to 12 hours of 949 ng·h/mL. Following systemic uptake, crisaborole is rapidly and extensively metabolized into inactive metabolites AN7602 and AN8323, primarily via hydrolysis and oxidation. Steady-state plasma concentrations of crisaborole and its metabolites are reached by day 8, and the three compounds are eliminated through renal excretion as metabolites. In vitro studies suggest that crisaborole, AN7602, and AN8323 are unlikely to induce or inhibit cytochrome P450 (CYP) isoenzymes under clinical use conditions. AN8323 was found to be a moderate inhibitor of CYP2C8 and 2C9 and a weak inhibitor of CYP1A2 and 2B6 but did not inhibit the activities of CYP2C19, 2D6, and 3A4. No drug interaction potential was observed between crisaborole and warfarin in a clinical study. 2
Therapeutic Efficacy
Crisaborole ointment 2% has shown therapeutic efficacy in the treatment of mild to moderate atopic dermatitis, as demonstrated by two phase III studies (AD-301 and AD-302). These studies were randomized, double-blind, vehicle-controlled, multicenter trials that included patients aged 2 years and older with clinically diagnosed atopic dermatitis. Patients with an Investigator's Static Global Assessment (ISGA) score of 2 or 3 (indicating mild or moderate severity) and a minimum of 5% body surface area affected were eligible for enrollment. During the 28-day treatment period, patients were instructed to apply either crisaborole ointment 2% or a vehicle control twice daily to all affected areas. The primary efficacy endpoint was the proportion of patients at day 29 who achieved success, defined as an ISGA grade of clear or almost clear with a 2-grade improvement from baseline. Secondary efficacy endpoints included the proportion of patients with an ISGA grade of clear or almost clear at day 29 and the time to success. The results showed that crisaborole ointment 2% was significantly more effective than the vehicle control in improving disease severity and symptoms of atopic dermatitis. The success rates at day 29 were higher with crisaborole ointment 2% compared to the vehicle control. The treatment effect was observed as early as day 8 and sustained throughout the treatment period. The ointment also demonstrated improvements in other ISGA-related endpoints, such as erythema, exudation, excoriation, induration/papulation, and lichenification. Additionally, patients treated with crisaborole ointment 2% experienced early and sustained improvement in pruritus (itching). In conclusion, topical therapy with crisaborole ointment 2% has been shown to effectively treat mild to moderate atopic dermatitis, providing relief from symptoms and improving disease severity. 3
Reference
1. Freund YR, Akama T, Alley MR, et al. Boron-based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012;586(19):3410–3414.
2. Anacor Pharmaceuticals Inc. EUCRISATM (crisaborole) ointment, 2%, for topical use: US prescribing information. 2016.
3. Hoy SM. Crisaborole Ointment 2%: A Review in Mild to Moderate Atopic Dermatitis. Am J Clin Dermatol. 2017;18(6):837-843.
- Related articles
- Related Qustion
- Crisaborole ointment: Indications, Safety and Efficacy Dec 16, 2024
Crisaborole has broad-spectrum anti-inflammatory activity by mainly targeting phosphodiesterase 4 (PDE4) enzyme that is a key regulator of inflammatory cytokine production.
- Therapeutic potential and mechanism of action of Crisaborole Nov 9, 2023
Crisaborole is a non-steroidal topical medication for the treatment of mild-moderate atopic dermatitis.
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Dec 16,2024Biochemical EngineeringEntecavir is an effective antiviral for chronic hepatitis B, inhibiting viral replication. Side effects are mild, and resistance can occur in non-naive patients.....
Feb 6,2024APICrisaborole
906673-24-3You may like
- AN 2728
-
- $63.00 / 1g
- 2025-10-26
- CAS:906673-24-3
- Min. Order: 1g
- Purity: 0.98
- Supply Ability: 10kg
- 4-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yl)oxy)benzonitrile
-
![906673-24-3 4-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yl)oxy)benzonitrile](/ProductImageEN/2022-03/Small/bc95d95c-b5e4-4e2d-a473-73f00db1cbd7.jpg)
- $2.00 / 1KG
- 2025-10-25
- CAS:906673-24-3
- Min. Order: 1KG
- Purity: 99.99%
- Supply Ability: 500KG
- Crisaborole
-

- $0.00 / 1kg
- 2025-10-24
- CAS:906673-24-3
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 500kg