Crystal structure of COBALT PHOSPHIDE and its derivatives
Apr 23,2024
Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced hydrogen evolution in alkaline solution
Transition metal phosphides exhibit promising catalytic performance for the hydrogen evolution reaction (HER); however their surface structure evolution during electrochemical operation has rarely been studied. In this work, we investigate the surface reconstruction of CoP nanosheets by an in situ electrochemical activation method. After remodeling, CoP nanosheets experience an irreversible and significant evolution of the morphology and composition, and low-valence Co complexes consisting of Co(OH)x species are formed on the surface of CoP nanosheets, and they largely accelerate the dissociation of water. Benefiting from the synergistic effect of CoP and Co(OH)x, the working electrode shows a remarkably enhanced HER activity of 100 mV at 10 mA cm−2 with a Tafel slope of 76 mV dec−1, which is better than that of most transition metal phosphide catalysts.
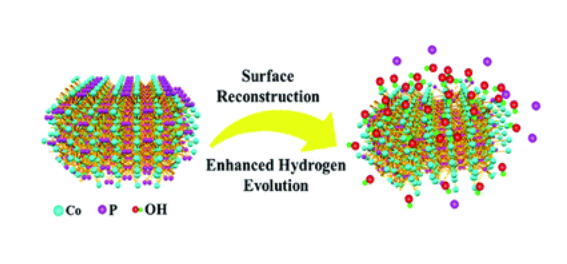
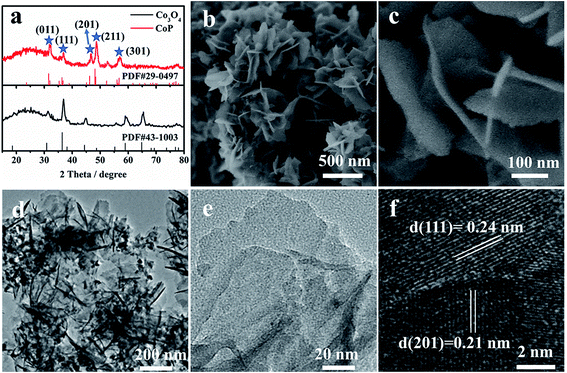
CoP nanosheets are prepared by a topological transformation using Co-oxides as the precursor followed by a phosphidation process in a reductive environment (Fig. S1†). X-ray diffraction (XRD) patterns indicate the successful transformation of the cobalt oxide precursor to crystalline CoP (PDF 29-0497) (Fig. 1a). The scanning electron microscopy (SEM) image of CoP products demonstrates the interconnected nanosheet morphology (Fig. 1b). Fig. 1c clearly shows an average size of 200 nm width and less than 5 nm thickness for these nanosheets. Moreover, the elements including Co, P, and O are homogeneously dispersed according to the elemental mapping images (Fig. S2c†). From the corresponding energy-dispersive X-ray spectrometry (EDX) result (Fig. S2e†), the elemental content of Co and P is 21.1 at% and 19.2 at%, respectively, demonstrating that the stoichiometric ratio of Co to P is close to 1 : 1. Transmission electron microscopy (TEM) images present a nanosheet structure (Fig. 1d), while the thickness is estimated to be ∼4 nm, consistent with the SEM observation (Fig. 1c). The well-resolved lattice fringes with interplanar spacings of 0.24 nm and 0.21 nm correspond to the (210) and (201) planes of crystalline CoP, respectively (Fig. 1f).
Preparation and crystal structure of the holmium cobalt phosphide HoCo3P2 and isotypic lanthanoid cobalt phosphides
The new compound HoCo3P2 was prepared by reaction of the elemental components in a tin flux. Its crystal structure was determined from single-crystal X-ray data and refined to a residual of R = 0.055 for 2441 unique structure factors and 50 variable parameters. It is of a new orthorhombic type, space group Pmmn-D2h13 with Z = 6 formula units per cell, and the lattice constants a = 10.560(2)Å, b = 3.688(1) Å, c = 12.233(2) Å, . The structure may be viewed as built up by parallel corner-sharing phosphorus-centered metal prisms and thus can be considered as closely related to several other structures, all with a metal:metalloid ratio of 2:1, namely, the structures of Fe2P, Zr2Fe12P7, Hf2Co4P3, TiNiSi, YNi5Si3, YCo5P3, and LaCo5P3. These structures, however, show a considerable variety in the coordination polyhedra of the metal atoms. The compounds LnCo3P2 (Ln ≡ Tb, Dy, Er, Tm, Yb, Lu) are isotypic with HoCo3P2.
References:
[1] W. JEITSCHKO U. J. Preparation and crystal structure of the holmium cobalt phosphide HoCo3P2 and isotypic lanthanoid cobalt phosphides[J]. Journal of The Less Common Metals, 1985. DOI:10.1016/0022-5088(85)90342-X.
[2] SU L, CUI X, HE T, et al. Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced hydrogen evolution in alkaline solution†[J]. Chemical Science, 2018, 7: 2019-2024. DOI:10.1039/C8SC04589E.
- Related articles
- Related Qustion
Acetyl chloride is a powerful acetylating agent. It is used in the manufacture of aspirin, acetaminophen, and acetanilide. Liquid crystal compositions for optical display and memory devices frequently require acetyl chloride.....
Apr 24,2024Organic reagentsCOBALT PHOSPHIDE
12134-02-0You may like
COBALT PHOSPHIDE manufacturers
- COBALT PHOSPHIDE
-

- $1.00 / 1KG
- 2019-09-02
- CAS:12134-02-0
- Min. Order: 1KG
- Purity: 85.0-99.8%
- Supply Ability: 200kg





