Dapoxetine: Pharmacokinetics and Clinical Applications
Oct 28,2024
General Description
Dapoxetine, marketed as Priligy, is an effective treatment for premature ejaculation due to its rapid absorption, short half-life, and minimal impact on norepinephrine and dopamine. Dapoxetine's pharmacokinetic profile allows for swift onset of action and consistent plasma concentrations with multiple doses. Clinical trials have shown significant improvement in Intravaginal Ejaculation Latency Time (IELT) with both 30 mg and 60 mg doses, leading to higher patient satisfaction levels. While some subjects discontinued treatment due to perceived lack of efficacy and mild adverse events like nausea and dizziness, dapoxetine remains a promising option for on-demand treatment of PE, especially considering its low prevalence of adverse events and lack of significant drug interactions.

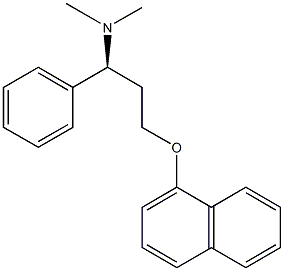
Figure 1. Dapoxetine
Pharmacokinetics1
Dapoxetine is a serotonin transporter inhibitor with unique physiochemical and pharmacokinetic properties, which was developed specifically for the treatment of PE. Dapoxetine was rapidly absorbed following oral administration, with peak plasma concentrations reached approximately 1 hour after dosing; by 24 hours, plasma concentrations had decreased to approximately 5% of the peak concentrations. Elimination of dapoxetine was rapid and biphasic, with an initial half-life of approximately 1.4 hours and a terminal half-life of approximately 20 hours. The 30- and 60-mg doses of dapoxetine exhibited dose proportional and time-invariant pharmacokinetics. Dapoxetine pharmacokinetics was unaffected by multiple dosing; steady-state was achieved after 4 daily doses, with modest accumulation (approximately 1.5-fold) after daily administration. In contrast, long-acting SSRI antidepressants, which are sometimes used to treat PE, exhibit significant accumulation. The dapoxetine metabolites desmethyldapoxetine and dapoxetine-N-oxide exhibited similar single- and multiple-dose pharmacokinetics. In addition, the metabolism of dapoxetine to desmethyldapoxetine and dapoxetine-N-oxide was not altered with multiple dosing, indicating that there is not a disproportionate accumulation or reduction of dapoxetine metabolites with multiple dosing, which would have the potential to affect the efficacy of the drug for the treatment of PE (ie, by effectively decreasing or increasing the concentration of dapoxetine, the primary pharmacologically active species).
While there are currently no pharmacologic agents indicated for the treatment of PE, SSRI antidepressants are sometimes prescribed because of their known side effect of delayed ejaculation. Two SSRIs, paroxetine and fluoxetine, have been studied in small trials to determine their benefit in the treatment of PE; their pharmacokinetic profiles are very different from that of dapoxetine. Fluoxetine absorption is slow compared to dapoxetine—maximal plasma concentrations of fluoxetine are reached 6 to 8 hours after oral administration. Fluoxetine has a long half-life of 1 to 4 days, and its active metabolite norfluoxetine has an even longer half-life of 7 to 15 days; accordingly, 2 to 4 weeks may be required to achieve steady state. The pharmacokinetics of fluoxetine is nonlinear, and multiple dosing results in disproportionate increases in Cmax and t1/2. Paroxetine is also slowly absorbed, requiring 5 hours to reach maximal concentrations. Elimination of paroxetine varies greatly with increased doses or multiple dosing; administration of paroxetine 20 mg for 15 days increases the half-life from 16.4 to 18.3 hours and from 9.8 to 21.0 hours with repeated once-daily dosing of paroxetine 30 mg. The AUC of paroxetine increases even more dramatically with multiple dosing, from 191 to 1481 ng•h/mL (at the 20-mg dose). Paroxetine also exhibits nonlinear pharmacokinetics and requires 7 to 14 days to reach steady state, while exhibiting approximately 8-fold accumulation.
Clinical Applications2
Because of its rapid action and short half-life, the on-demand use of dapoxetine makes it a popular alternative for treating PE. Currently, dapoxetine is approved for the treatment of PE in over 50 countries. Several randomized controlled trials (RCTs) demonstrated the efficacy and safety of dapoxetine on more than 6,000 men with PE in over 25 countries. Integrated analysis of these phase III trials of dapoxetine demonstrate a significant increase in geometric mean IELT, from baseline (0.8 min) with 30 mg (2.0 min) and 60 mg (2.3 min) vs. placebo (1.3 min) at 12 weeks. In addition to IELT, both doses of dapoxetine improved patient reported outcome measures compared to placebo. Dapoxetine was comparably effective both in men with lifelong and acquired PE.
Despite these favorable outcomes, the results of the integrated analysis of the clinical dapoxetine trials revealed that 30.4% of the subjects included into the study discontinued, mostly due to lack of efficacy and personal reasons. These findings were in accordance with those of a recent report that demonstrated 20% of lifelong PE patients decided not to start dapoxetine treatment and almost 90% of the ones who initiated this therapy discontinued within one year because the beneficial effect were below expectations (24.4%), cost (22.1%), side effects (19.8%), loss of interest in sex 19.8%, and lack of efficacy 13.9%.
References:
[1] NISHIT B. MODI PHD. Single- and Multiple-Dose Pharmacokinetics of Dapoxetine Hydrochloride, a Novel Agent for the Treatment of Premature Ejaculation[J]. The Journal of Clinical Pharmacology, 2013, 46 3: 258-379. DOI:10.1177/0091270005284850.
[2] MARCEL D WALDINGER B O Dave H Schweitzer. Dapoxetine treatment of premature ejaculation.[J]. The Lancet, 2006, 368 9550. DOI:10.1016/S0140-6736(06)69773-0.
- Related articles
- Related Qustion
- Dapoxetine is an effective drug for the treatment of premature ejaculation Apr 11, 2022
Dapoxetine, a selective serotonin reuptake inhibitor, is the first oral pharmacological agent indicated for the treatment of men aged 18–64 years with premature ejaculation.
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringL-Glutamic acid is one of the 20-22 proteinogenic amino acids, and its codons are GAA and GAG.....
Dec 30,2024APIDapoxetine
119356-77-3You may like
- Dapoxetine hydrochloride
-

- 2025-12-14
- CAS:119356-77-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Dapoxetine
-

- $1.00 / 1kg
- 2025-12-12
- CAS:119356-77-3
- Min. Order: 0.10000000149011612kg
- Purity: 99%
- Supply Ability: 200KG
- Dapoxetine
-

- $10.00 / 1KG
- 2025-12-11
- CAS:119356-77-3
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt