Dehydroacetic Acid: Properties and Biological Activity
Aug 27,2024
General Description
Dehydroacetic acid is a white crystalline solid that is notable for its odorless nature and weakly acidic properties and exhibits notable antimicrobial and antifungal properties, particularly when used as a scaffold for synthesizing ligands and metal complexes. Research indicates that its copper(II) complex shows significant potency against Escherichia coli and Aspergillus niger. Additionally, the anticancer potential of dehydroacetic acid derivatives has been explored, with compounds demonstrating effective inhibition against cancer cell lines, such as GL261 and HeLa. Furthermore, dehydroacetic acid is being investigated for DNA targeting and photocleavage applications, enabling targeted gene regulation and potential advances in cancer therapies, underscoring its broad significance in medicinal chemistry.

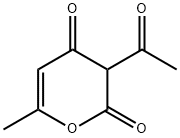
Figure 1. Dehydroacetic acid
Properties
Physical and Chemical Properties
Dehydroacetic acid, with the molecular formula C8H8O4, is a white crystalline solid that is notable for its odorless nature and weakly acidic properties. This compound is a derivative of pyrone and exhibits poor solubility in water. The chemical structure of dehydroacetic acid features both nucleophilic and electrophilic centers, which contribute to its reactivity. Specifically, the compound has highly nucleophilic sites at positions C2, C3, C4, and C6, while the acetyl group at C3 and C5 serve as electrophilic centers. This dual reactivity plays a significant role in the chemical behavior and applications of dehydroacetic acid. 1
Tautomerism and Industrial Preparation
Dehydroacetic acid exhibits tautomerism, existing in two forms: the enol form (3-acetyl-4-hydroxy-6-methyl-2H-pyran-2-one) and the keto form (3-acetyl-2-hydroxy-6-methyl-4H-pyran-2-one). These tautomers interconvert through a slower keto-enol tautomerism compared to other β-diketones. The industrial synthesis of dehydroacetic acid involves the base-catalyzed dimerization of diketene, utilizing catalysts such as pyridine, sodium acetate, and imidazole. The ability of dehydroacetic acid to undergo various structural modifications makes it a valuable compound for developing biologically active molecules, highlighting its significance in chemical and pharmaceutical research. 1
Biological Activity
Antimicrobial Activities
Dehydroacetic acid has demonstrated notable antimicrobial and antifungal properties when used as a scaffold for synthesizing various ligands and their metal complexes. Research by Abd-ElRazek et al. involved creating benzoyl acetohydrazone ligands based on dehydroacetic acid and testing their complexes with transition metal ions for biological activity. Among these, the copper(II) complex exhibited the highest antimicrobial potency, achieving a zone of inhibition of 18.3 mm against Escherichia coli. Similarly, another complex showed significant antifungal activity, with a zone of inhibition of 25 mm against Aspergillus niger. The structure-activity relationship (SAR) studies indicated that while the individual ligands based on dehydroacetic acid had limited biological activity, their complexation with metal ions significantly enhanced their antimicrobial efficacy. This demonstrates the potential of dehydroacetic acid derivatives in developing effective antimicrobial agents. 2
Anticancer Potential
The anticancer potential of dehydroacetic acid derivatives has been explored through the synthesis of various compounds and their evaluation in vitro. Mamidala et al. synthesized thiadiazines with a dehydroacetic acid backbone and tested their anticancer activity. The compound designated as 75 showed significant potency against cancerous GL261 cells with an IC50 value of 7.1 ± 1.3 µM, which was comparable to the commercially available drug WP1066 (IC50 = 4.91 µM). Molecular docking studies revealed that this compound binds effectively to the active site of STAT3, showing superior binding energy compared to WP1066. Furthermore, Ahmad et al. investigated dehydroacetic acid hydrazone-derived ketimines and their metal complexes, finding that the copper complex demonstrated a 97% inhibition rate against HeLa cervical cancer cells. These findings suggest that dehydroacetic acid-based compounds possess significant anticancer potential, particularly when modified and complexed with metal ions. 2
DNA Targeting and Photocleavage
Dehydroacetic acid is also of interest in the context of DNA targeting and photocleavage, which can be applied in medication development. Traditional photosensitizers have been used to study DNA damage by photosensitization, but recent advancements focus on targeted DNA systems with both natural and synthetic molecules. Dehydroacetic acid-based photocleavage agents, created by linking photosensitizers to molecules with high DNA affinity, enable specific DNA damage and targeted gene regulation. This strategy is valuable for developing novel therapies that can selectively induce DNA breaks at desired locations, potentially leading to advancements in gene expression modulation and treatment development. Additionally, research by Ghaith et al. demonstrated that dehydroacetic acid can be used to synthesize binary and fused pyrazole ring systems, which showed high anticancer potency in various cell lines. This indicates that dehydroacetic acid-based compounds have broad applications, from DNA targeting to effective cancer therapies. 2
References:
[1] ANSHUL GROVER. Dehydroacetic acid a privileged medicinal scaffold: A concise review[J]. Archiv der Pharmazie, 2023, 357 2. DOI:10.1002/ardp.202300512.
- Related articles
- Related Qustion
1,7-Dimethylxanthine is a naturally occurring alkaloid compound that can enhance alertness and reduce drowsiness.....
Feb 27,2025APIAdenosine terminates AV nodal-dependent arrhythmias like atrioventricular nodal reentrant tachycardia (AVNRT) and atrioventricular reentrant tachycardia (AVRT).....
Aug 27,2024Biochemical EngineeringDehydroacetic acid
520-45-6You may like
Dehydroacetic acid manufacturers
- Dehydroacetic acid
-

- 2025-10-30
- CAS:520-45-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Dehydroacetic acid
-

- $31.00 / 1g
- 2025-10-29
- CAS:520-45-6
- Min. Order:
- Purity: 99.51%
- Supply Ability: 10g
- Dehydroacetic acid
-

- $90.00 / 10g
- 2025-10-26
- CAS:520-45-6
- Min. Order:
- Purity: 99.51%
- Supply Ability: 10g