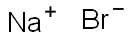Demystifying Sodium bromide: An In-depth look at its Applications and Implications
Oct 28,2024
Introduction
Sodium bromide is an inorganic compound with the formula NaBr. It is a high-melting white, crystalline solid that resembles sodium chloride. It is a widely used source of the bromide ion and has many applications.

Peculiarity
(1) Physical properties
Sodium bromide usually appears as a white crystalline or granular solid. In the form of anhydrous sodium bromide, it is a colorless crystal. It has good solubility in water and can quickly dissolve and form sodium bromide aqueous solution. Its solubility increases with the increase in temperature. From the melting point and boiling point, it has a melting point of about 755°C and a boiling point of about 1390°C. It can melt into a liquid state at high temperatures.1
(2) Chemical properties
Sodium bromide is an alkaline compound that reacts with acids to form corresponding salts and water. For example, when it reacts with hydrochloric acid, sodium chloride and hydrogen bromide are formed. It can be oxidized under certain conditions. For example, when it comes into contact with a reducing agent, an oxidation reaction can occur. It can be used as a source of bromine in chemical reactions. In organic synthesis, it is often used as an introducer of bromine atoms and participates in many organic reactions such as substitution reactions. In addition, it has a certain toxicity, and excessive intake may lead to poisoning. Therefore, the dosage needs to be carefully controlled during use, and direct contact with the skin or inhalation of its dust should be avoided.2
Application and challenge
Its lattice structure and water solubility make sodium bromide a common ingredient in a variety of chemical processes and industrial applications. One of its main functions is as a precursor to other bromine compounds and is essential in synthetic drugs, flame retardants, and agricultural chemicals. The pharmaceutical industry in particular relies on NaBr as a key ingredient in drugs used to treat epilepsy and as a sedative in veterinary medicine.3
In addition to medical uses, it also plays a vital role in oil and gas extraction. Its ability to increase the viscosity of drilling fluids makes it an indispensable component of the well-drilling process. In addition, sodium bromide is useful in water treatment facilities, where its properties help to disinfect and control algae. This versatility underscores the importance of NaBr in a range of industries, from healthcare to energy production to environmental management.
However, the widespread use of sodium bromide has also caused environmental problems. After entering natural water, bromide ions can react with chlorine and other disinfectants to form brominated disinfection byproducts (DBPs). These DBPS have been associated with adverse health effects, including potential carcinogenicity. Therefore, the discharge of sodium bromide-containing wastewater needs to be carefully regulated and treated to mitigate its impact on the environment.4
In recent years, research efforts have focused on developing alternative methods of bromine production to reduce reliance on NaBr and minimize its environmental impact. Electrochemical processes and catalytic bromination reactions are promising approaches for sustainable bromine synthesis. In addition, advances in wastewater treatment technology aim to effectively remove bromide ions from industrial wastewater, thereby inhibiting the formation of harmful DBPs.
References:
[1] Y-S LIN J L S B M Auer. Water structure, dynamics, and vibrational spectroscopy in sodium bromide solutions.[J]. Journal of Chemical Physics, 2009, 131 14. DOI:10.1063/1.3242083.
[2] W. R. HAAF G C. The crystal structure of sodium bromide dihydrate[J]. Acta Crystallographica, 1964, 1 1. DOI:10.1107/S0365110X64001797.
[3] ADDISON W L. The Use of Sodium Chloride, Potassium Chloride, Sodium Bromide, and Potassium Bromide in Cases of Arterial Hypertension which are Amenable to Potassium Chloride.[J]. Canadian Medical Association journal, 1928, 18 3.
[4] J.H. CANTON E A M M S P W Wester. Study on the toxicity of sodium bromide to different freshwater organisms[J]. Food and Chemical Toxicology, 1983, 21 4: 355-526. DOI:10.1016/0278-6915(83)90090-X.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringTween(R) 20, a widely used non-ionic surfactant, is suitable for preparing O/W emulsions.....
Dec 30,2024APISodium bromide
7647-15-6You may like
- Sodium bromide
-

- 2025-11-15
- CAS:7647-15-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Sodium bromide
-
- $10.00 / 25kg
- 2025-11-14
- CAS:7647-15-6
- Min. Order: 1kg
- Purity: 99.5%
- Supply Ability: 100
- Sodium bromide
-

- $0.00 / 25KG
- 2025-09-16
- CAS:7647-15-6
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50000KG/month