Dichloroethane: A Versatile Solvent in Modern Chemistry
May 23,2024
Introduction
Dichloroethane, often abbreviated as DCE, encompasses several isomers, with 1,2-dichloroethane (ethylene dichloride, EDC) and 1,1-dichloroethane being the most significant. This colorless liquid, characterized by its chloroform-like odor, plays a crucial role primarily in the production of vinyl chloride, the precursor to polyvinyl chloride (PVC). PVC is among the most widespread plastics globally, used extensively across various industries due to its versatility and durability. The significance of DCE extends beyond PVC production, as it serves as a critical solvent and intermediate in numerous chemical processes.

Figure 1 Characteristics of Dichloroethane
Synthesis
The primary industrial route to 1,2-dichloroethane involves the direct chlorination of ethylene, facilitated by iron(III) chloride (FeCl3) as a catalyst. This highly exothermic reaction requires precise control of temperature and pressure to maximize yield and minimize by-product formation. Additionally, the process must be constantly monitored to ensure safety due to the reactive nature of the materials involved. Alternatively, oxychlorination represents another significant synthetic route. In this method, hydrogen chloride (HCl) and oxygen are reacted with ethylene in the presence of a copper chloride (CuCl2) catalyst. This technique not only helps recycle HCl but also significantly reduces chlorine consumption, presenting a more sustainable approach to DCE production.
Main Components
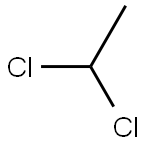
Dichloroethane primarily consists of carbon, hydrogen, and chlorine atoms. Specifically, in 1,2-dichloroethane, two chlorine atoms are bonded to adjacent carbon atoms in the ethylene molecule, creating a V-shaped molecular geometry due to the sp3 hybridization of the carbon atoms. This unique structural arrangement is pivotal for its chemical reactivity and versatility in various syntheses. The electron-rich chlorines enhance its reactivity with other compounds, making it an essential intermediate in numerous industrial processes. Moreover, the polarity and steric properties of the molecule influenced by this arrangement facilitate its interaction with other reactants, which is critical in applications such as solvent extraction and polymer production, further broadening its utility in the chemical industry.
Uses
The predominant use of 1,2-dichloroethane (DCE) is in the production of vinyl chloride through a process called dehydrochlorination, which occurs at high temperatures. Vinyl chloride is the primary ingredient in the manufacture of polyvinyl chloride (PVC), a plastic extensively used in construction, automotive, and electrical industries for its durability, resistance to environmental degradation, and excellent insulation properties. Beyond its role in PVC production, DCE serves as a versatile intermediate in synthesizing other vital chemicals. It is also employed as an effective solvent in various applications, including cleaning and degreasing operations in industrial settings. DCE's ability to dissolve a broad spectrum of organic compounds makes it invaluable in promoting chemical reactions, particularly in organic synthesis. Its use in the pharmaceutical industry as a solvent and in the production of adhesives and synthetic fibers underscores its versatility and critical role in modern manufacturing processes.
Storage Methods
Storing dichloroethane requires careful consideration due to its volatility and reactivity. It must be kept in cool, dry conditions away from direct sunlight and heat sources to prevent decomposition and minimize the risk of fire and explosion. Stainless steel or aluminum tanks are preferred for bulk storage, equipped with inert gas blanketing to prevent exposure to air and moisture. Additionally, proper ventilation must be maintained in storage areas to avoid the accumulation of vapors, which are heavier than air and can create hazardous environments.
Conclusion
Dichloroethane plays an indispensable role in the chemical industry, especially in the synthesis of PVC. Understanding its properties, synthesis, and applications allows industry professionals to utilize this chemical safely and effectively. As the industry continues to evolve, the efficient and environmentally responsible handling of dichloroethane remains a priority, ensuring its continued utility in industrial applications while mitigating health and environmental impacts. The ongoing research and development in the area of catalysis and green chemistry are expected to enhance the production processes of dichloroethane, further solidifying its position in modern industrial chemistry.
![]() References
References
[1]Ollis, David F., et al. "Heterogeneous photoassisted catalysis: conversions of perchloroethylene, dichloroethane, chloroacetic acids, and chlorobenzenes."Journal of Catalysis88.1 (1984): 89-96.
[2]**a Shikai, et al. "Competitive adsorption of water vapor with VOCs dichloroethane, ethyl acetate and benzene on MIL-101 (Cr) in a humid atmosphere."RSC Advances5.3 (2015): 1827-1834.
- Related articles
- Related Qustion
L-Leucine, a critical player in the biochemistry of life, stands out as one of the nine essential amino acids that humans must obtain through their diet.....
May 23,2024APILidocaine remains a cornerstone in medical treatments involving anesthesia and pain management due to its effective blocking of nerve signal transmissions.....
May 23,2024APIDichloroethane
1300-21-6You may like
- Dichloroethane
-

- $50.00 / 1kg
- 2024-06-26
- CAS:1300-21-6
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 500
- Dichloroethane
-

- $18.00 / 1KG
- 2024-06-18
- CAS:
- Min. Order: 1KG
- Purity: 99.99%
- Supply Ability: 20 tons
- Dichloroethane
-

- $490.00 / 1T
- 2024-05-08
- CAS:1300-21-6
- Min. Order: 1T
- Purity: 98%
- Supply Ability: 20T