Exploring Metformin Hydrochloride: From Chemical Structure to Clinical Applications
Jun 17,2024
Introduction
Metformin hydrochloride stands as a cornerstone in the treatment of type 2 diabetes mellitus, showcasing a remarkable journey from traditional herbal medicine to a staple in modern pharmaceuticals. Originally derived from the French lilac or goat's rue, a plant known for its potent anti-diabetic properties, metformin was first synthesized in the 1920s but gained clinical prominence several decades later. This transition marks a pivotal moment in medical history, transforming an ancient natural remedy into a critical element of contemporary diabetes management strategies. Today, metformin hydrochloride is not only celebrated for its efficacy and safety profile but also for its role in addressing the global diabetes epidemic by providing an affordable and accessible treatment option.

Figure 1 Characteristics of Metformin hydrochloride
Properties of Metformin Hydrochloride
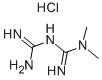
Metformin hydrochloride (C4H11N5 • HCl) is a hydrochloride salt form of metformin, a biguanide class of antidiabetic medications that primarily helps in lowering blood glucose levels. It is a white to off-white crystalline compound that is soluble in water, slightly soluble in alcohol, and practically insoluble in acetone and ether. Its molecular weight is 165.63 g/mol. Metformin hydrochloride exhibits a unique therapeutic profile as it does not cause hypoglycemia unlike other anti-diabetic drugs; instead, it reduces basal and postprandial plasma glucose.
Main Components
The primary component of metformin hydrochloride is metformin, a dimethylbiguanide. Its chemical structure includes two linked guanidine rings which are responsible for its pharmacological action. Metformin is synthesized from dichloroacetamide and cyanoguanidine, under specific alkaline conditions which lead to the formation of the biguanide structure. The hydrochloride salt is added to increase its solubility and stability, facilitating easier formulation into tablets and solutions. This chemical modification enhances the drug's bioavailability, allowing it to be more effectively absorbed by the body. Additionally, the ionic nature of the hydrochloride salt helps in the controlled release of metformin, thus optimizing its therapeutic effects and minimizing side effects.
Uses of Metformin Hydrochloride
Metformin hydrochloride is primarily used in the management of type 2 diabetes mellitus. It enhances the sensitivity of liver, muscle, and fat tissue to insulin, thereby improving glucose uptake and decreasing insulin resistance. It also inhibits hepatic gluconeogenesis, the production of glucose from non-carbohydrate substrates in the liver. Moreover, metformin hydrochloride has been investigated for its benefits in various other conditions, including polycystic ovary syndrome (PCOS), non-alcoholic fatty liver disease, and as a potential anti-aging agent. It reduces cardiovascular mortality and morbidity, which is a significant advantage over other antidiabetic drugs. Additionally, ongoing research is exploring its effectiveness in reducing cancer risk and improving cognitive function, potentially broadening its therapeutic reach beyond traditional metabolic disorders. This versatility underlines its potential as a multifaceted therapeutic agent in a variety of clinical settings.
Storage Methods
Proper storage of metformin hydrochloride is crucial for maintaining its efficacy and safety. The drug should be stored at room temperature, typically between 20°C to 25°C, in a tightly closed container to protect it from moisture and light. It should also be kept out of reach of children to prevent accidental ingestion. The stability of metformin hydrochloride is also a function of pH, and it is most stable at pH 2.5 to 6.5; therefore, solutions prepared for administration should be within this pH range to avoid degradation of the active ingredient.
Conclusion
Metformin hydrochloride continues to be a subject of extensive research and discussion among scientific communities, due to its broad therapeutic applications and favorable safety profile. Understanding its properties, composition, and correct storage methods is crucial for chemists and pharmaceutical scientists who develop, prescribe, and dispense this vital medication. As we continue to uncover the full potential of metformin hydrochloride, it remains a pivotal component in the management of not only diabetes but also other metabolic and endocrine disorders. This exploration not only enhances our understanding of metformin hydrochloride but also emphasizes the significance of chemistry in the development and optimization of pharmaceutical agents.
![]() References
References
[1]Setter, Stephen M., et al. "Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy."Clinical therapeutics25.12 (2003): 2991-3026.
[2]Klepser, Teresa B., and Michael W. Kelly. "Metformin hydrochloride: an antihyperglycemic agent."American journal of health-system pharmacy54.8 (1997): 893-903.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical Engineering4-Methoxybenzoic acid, commonly referred to as p-Anisic acid, represents a significant compound in the realm of organic chemistry.....
Jun 17,2024APIMetformin hydrochloride
1115-70-4You may like
Metformin hydrochloride manufacturers
- 1,1-Dimethylbiguanide hydrochloride
-

- $5.00/ KG
- 2025-12-16
- CAS:1115-70-4
- Min. Order: 1KG
- Purity: 99% hplc
- Supply Ability: 500TONS
- Metformin hydrochloride
-

- $980.00/ kg
- 2025-12-16
- CAS:1115-70-4
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Metformin hydrochloride
-

- $77.00 / 500mg
- 2025-12-16
- CAS:1115-70-4
- Min. Order:
- Purity: 99.97%
- Supply Ability: 10g