FMOC-LYS(BOC)(ME)-OH: Synthesis and Application
Dec 12,2022
General description
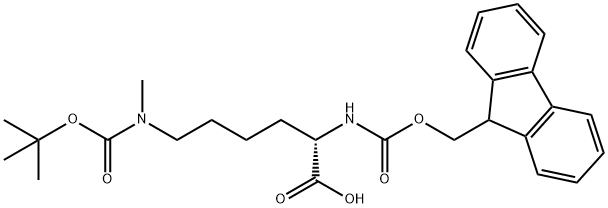
FMOC-LYS(BOC)(ME)-OH is an amino acid derivative, which can be used as a medical intermediate.
Synthetic routes
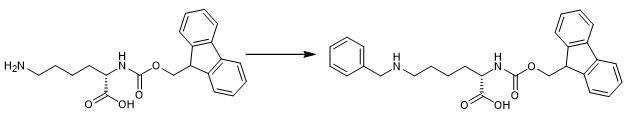
Fig. 1 The synthetic step 1 of FMOC-LYS(BOC)(ME)-OH.
Place 400 mL EtOH over 3? molecular sieves overnight at room temperature. Dissolve 40 g (83 mmol) of the trifluoroacetate salt of Fmoc-Lys-OH in the ethanol and add 10mL (98 mmol) benzaldehyde. Stir the solution overnight at room temperature under argon balloon. Add 6.3 g (99 mmol) NaCNBH3 in three portions [1].
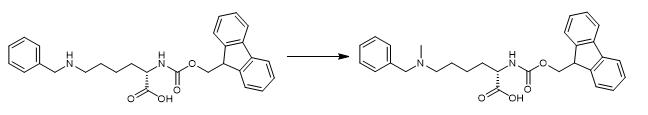
Fig. 2 The synthetic step 2 of FMOC-LYS(BOC)(ME)-OH.
Add 12 mL (161 mmol) of 37% aqueous formaldehyde and add again the addition of 7.3 g (62.84 mmol) NaCNBH3 in four portions. Stir the reaction mixture for one hour stirring at room temperature. Pour the reaction over ice and add 6M HCl (in a well-ventilated hood) to a pH of 2 by litmus paper. Filter the solids and rinse with 0.5 M HCl. Add 200cc silica to the filtrate and remove the solvent by rotary evaporation and vacuum. Pack 3000cc of silica in 7.5% MeOH/DCM. Add the crude mixture which is adsorbed on silica to a plug of 7.5% MeOH/DCM at the top of the column. Add sodium sulfate on top and elute the column with 3L 7.5% MeOH/DCM and then 10% MeOH/DCM until all the di-benzylated impurity is eluted. Increase the MeOH percentage from 10 to 35% until all product is eluted with minimal di-methyl impurity. Remove solvent from product fractions. Remove the silica by reverse-phase chromatography on Combi-Flash, with a MeCN/H2O eluent. Remove MeCN from the pure fractions and lypphilize the water [1].
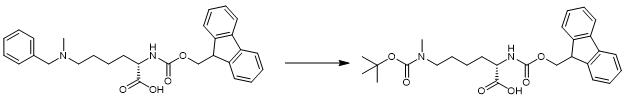
Fig. 3 The synthetic step 3 of FMOC-LYS(BOC)(ME)-OH.
Add 0.75 g (1.47 mmol) of the hydrochloride salt of Fmoc-Lys (Bn, Me) -OH, 0.43 g (2.0 mmol) di-tert-butyl dicarbonate, and 75 mg (0.070 mmol) 10% Pd/C to 5 mL 1, 2-dichloroethane. Sparge the reaction mixture twice with a H2 balloon. Add 620 μL N, N-diisopropylethylamine (3.56 mmol) and stir the reaction mixture under H2 balloon at room temperature overnight. Filter the Pd/C and wash the organic filtrate twice with 1 M HCl, once with saturated sodium bicarbonate solution, and once with brine. Dry the washed organics over sodium sulfate and filter. Remove the solvents by rotary evaporation and vacuum. Purify the product by reverse-phase chromatography on Combi-Flash, using a MeCN/H2O gradient. Remove MeCN from the pure fractions and lyophilizethe water [1].
Application
Optimization of a synthetic receptor for dimethyllysine
In the design of small mol. receptors for polar guests, much inspiration has been taken from proteins that have adapted effective ways to selectively bind polar mols. in aq. environments. Nonetheless, mol. recognition of hydrophilic guests in water by synthetic receptors remains a challenging task. Dimethyllysine can be synthesized by FMOC-LYS(BOC)(ME)-OH. Here we report a new synthetic receptor, A2I, with improved affinity and selectivity for a biol. important polar guest, dimethyllysine (Kme2). A2I was prepd. via redesign of a small mol. receptor (A2B) that preferentially binds trimethyllysine (Kme3) using dynamic combinatorial chem. (DCC). We designed a new biphenyl-2,6-dicarboxylate monomer, I, with the goal of creating a buried salt bridge with Kme2 inside a synthetic receptor. Indeed, incorporation of I into the receptor A2I resulted in a receptor with 32-fold enhancement in binding affinity, which represents the highest affinity receptor for Kme2 in the context of a peptide to date and is tighter than most Kme2 reader proteins. It also exhibits a ~2.5-fold increase in preference for Kme2 vs. Kme3 relative to the parent receptor, A2B. This work provides insight into effective strategies for binding hydrophilic, cationic guests in water and is an encouraging result toward a synthetic receptor that selectively binds Kme2 over other methylation states of lysine [2].
Effect on the RNA recognition and cellular uptake of Tat-derived peptides
The 2 Lys residues in the HIV virus trans-activator of transcription protein (HIV Tat protein) basic region (residues 47-57) are crucial for 2 biol. activities: RNA recognition and cellular uptake. Since the post-translational modifications of these 2 Lys residues affect the biol. function of the Tat protein, the authors investigated the effect of methylation and acetylation of Lys-50 and Lys-51 residues in Tat-derived peptides on the 2 biol. activities.Tat-derived peptides can be synthesized by FMOC-LYS(BOC)(ME)-OH. Tat-derived peptides, in which each Lys residue was replaced with a methylated- or acetylated-Lys, were synthesized by solid-phase peptide synthesis. TAR RNA recognition of the peptides was studied by electrophoretic mobility shift assays. Cellular uptake of the peptides into Jurkat cells was detd. by flow cytometry. The results showed that acetylation of either Lys residue attenuated both biol. activities. In contrast, the effect of Lys methylation on the bioactivities depended on position and no. of Me groups. These findings should be useful for the development of functional mols. contg. ammonium groups for RNA recognition to affect biol. processes and for cellular uptake for drug delivery [3].
Contributions of pocket depth and electrostatic interactions
Dynamic combinatorial chem. was used to generate a set of receptors for peptides contg. methylated lysine (KMen, n = 0-3) and study the contribution of electrostatic effects and pocket depth to binding affinity and selectivity. Peptides contg. methylated lysine (KMen, n = 0-3) can be synthesized by FMOC-LYS(BOC)(ME)-OH. We found that changing the location of a carboxylate resulted in an increase in preference for KMe2, presumably based on ability to form a salt bridge with KMe2. The no. of charged groups on either the receptor or peptide guest systematically varied the binding affinities to all guests by approx. 1-1.5 kcal mol-1, with little influence on selectivity. Lastly, formation of a deeper pocket led to both increased affinity and selectivity for KMe3 over the lower methylation states. From these studies, we identified that the tightest binder was a receptor with greater net charge, with a Kd of 0.2 μM, and the receptor with the highest selectivity was the one with the deepest pocket, providing 14-fold selectivity between KMe3 and KMe2 and a Kd for KMe3 of 0.3 μM. This work provides key insights into approaches to improve binding affinity and selectivity in water, while also demonstrating the versatility of dynamic combinatorial chem. for rapidly exploring the impact of subtle changes in receptor functionality on mol. recognition in water [4].
Development and mechanistic studies of an optimized receptor for trimethyllysine
Trimethyllysine (KMe3) can be synthesized by FMOC-LYS(BOC)(ME)-OH. A new small mol. receptor, A2N, has been identified that binds specifically to KMe3 with sub-micr
- Related articles
- Related Qustion
- Fmoc-Lys(Boc)(Me)-OH: Applications in Synthesis of Histone Tail Peptides and its Concise Preparation Method Apr 18, 2024
Fmoc-Lys(Boc)(Me)-OH streamlines peptide synthesis, enabling precise integration and facilitating studies on histone modifications with high yields and stability.
FMOC-LYS(BOC)(ME)-OH
951695-85-5You may like
FMOC-LYS(BOC)(ME)-OH manufacturers
- FMOC-LYS(BOC)(ME)-OH
-
- $1.00 / 1KG
- 2019-07-06
- CAS:951695-85-5
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20kg