General Introduction of Hydroxyacids
Mar 3,2022
Introduction
According to their chemical structure, hydroxyacids correspond to organic carboxylic acids characterized as the α- and β-types (AHA and BHA) according to the position of the hydroxyl group (OH) with regard to the acid (COOH) moiety. Globally, both categories exhibit almost similar biologic effects. These compounds are available worldwide, and likely have been for centuries, as dermatologic and cosmetic ingredients. Their acceptance by dermatologists, other physicians, cosmetologists, and consumers contrasts with the limited independent, well-controlled studies demonstrating their mechanisms of action and their long-term beneficial effects.
Health care and cosmetic regulations differ among continents and countries although skin biology is similar worldwide. Many physicians consider that some respective legal definitions of drugs and cosmetics are outdated and unworkable. It seems obvious that many environmental threats and topical products may produce some biologic effect on the skin. Hence, a series of cosmetics should be viewed as skin physiology modulators. Should they all be classified as real bioactive agents or remedial cosmetics? This is a matter of definition because bioactivity differs by several degrees of magnitude among product categories. In cosmetology, there is a huge difference between decorative formulations, suppletive compounds, and active physiology modulators.1 Many cosmetic products (makeup, hair styling, hygiene) do not require any biologic action despite being very efficient in terms of achievement of aesthetic needs. Sunscreens, for example, fit within such consideration: they do not interfere, per se, with skin physiology, although remain highly efficient in screening much harmful ultraviolet (UV) radiation from the skin. The very words “activity” (to induce an action) and “efficacy” (to induce an effect) have to be well differentiated.
There is vivid controversy about the concept of cosmeceuticals, designing compounds that fall between cosmetics and pharmaceuticals. This concept received immediate acceptance by some people. However, many corporate leaders contend that cosmeceuticals are neither scientifically sensible nor juridically necessary. In fact, with the exception of cosmeceuticals in the United States and “quasidrugs” in Japan, national regulatory agencies have not positively recognized the cosmeceutical class. The lack of a clear-cut of clarification risks having negative effects. Some products could be potentially forbidden although they are valuable in cosmetology. The opposite is also true, because some products are used in cosmetology without adequate evaluation of their potential biologic effects. One example is provided by the widespread use of AHAs and BHAs. Despite their obvious anti-xerotic and their peeling effects at given concentrations, there is little information available about their general toxicity and ancillary biologic effects. However, one should bear in mind the potential toxic effects of hydroxyacids. One example is given by O-hydroxybenzoic acid (salicylic acid) in the case of high percutaneous absorption, particularly in children.
Chemical Structure and Natural Sources of AHAs
AHAs range from simple aliphatic compounds to complex molecules. Many of these compounds were originally derived from natural sources, and thus are commonly referred to as fruit acids. However, a number of synthetic sources provide access to structural analogs. Most AHAs used in dermatology and cosmetology are currently produced by chemical synthesis. They are characterized in chemical groups based on the number of carboxylic groups (Table 8.1). According to their configuration, AHAs are present under different stereoisomeric structures called enantiomers “l” and “d” or “R” and “S.” Some of the common AHAs occur naturally in an enantiomerically enriched form, and both enantiomers may be available.
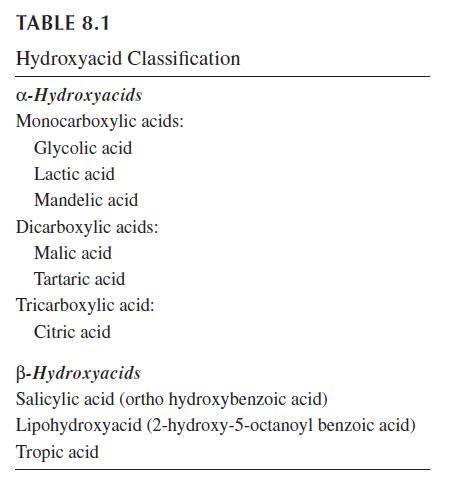
Glycolic acid (2-hydroxyethanoic acid) is a component of sugar cane juice. Lactic acid (2-hydroxypropanoic acid) was first isolated in 1780. The l-lactic acid produced by the microorganism Lactobacillus is responsible for the taste and odor of sour milk. It is, in addition, an end product of the anaerobic metabolism of the epidermis, present onto the skin surface and in eccrine sweat at low concentration. The other enantiomer d-lactic acid, also called sarcolactic acid, is formed during anaerobic muscular contraction, and is also found in apples, ergot, digitale, opium, and tomatoes. Mandelic acid (2-hydroxy-2-phenylethanoic acid) is obtained from hydrolysis of bitter almond extracts. Malic acid (2-hydroxy-1,4-butanedioic acid) was first isolated from unripened apples in 1785. Tartaric acid (2,3-hydroxy-1,4-butanedioic acid) was first isolated in 1769. It is widely distributed in plants, particularly in grapes and the lees of wine. Citric acid (2-hydroxy-1,2,3-propanetricarboxylic acid) was first isolated from lemon juice in 1784. It is also found in pineapples and other citrus fruits.
Biologic Activities of Hydroxyacid
A number of biologic aspects of the hydroxyacid action remain unsettled because hasty conclusions were offered from uncontrolled studies. Hence, a wealth of hydroxyacid-enriched cosmetics are present on the market with unsubstantiated claims or little evidence of performance. In some instances, erroneous information and incorrect statements have flourished behind promotional objectives.
At least one facet of the hydroxyacid biologic activities is ascribed to the native acid strength of the compounds. This physicochemical characteristic is measured by the proton dissociation in solution, and is expressed by its pKa. The lower the pKa of a hydroxyacid, the stronger its acidic strength: a decrease of 1 pKa unit represents a tenfold increase in the acid strength. If the acid strength influences some of the biologic effects of hydroxyacids, it does not, however, correlate with the overall potency of the final topical formulation.
The pH of the formulations varies with both the nature of the hydroxyacid and its concentration. In order to avoid irritation as much as possible, it is desirable to design a cosmetic formulation with a pH close to the physiologic pH range of the skin. This may be achieved by partial neutralization and by adding an effective buffer. However, AHA products at neutral pH seem to exert little effect on skin.
In order to prevent misunderstandings and misstatements, the biologic activities of hydroxyacids should be evaluated with regard to their chemical structure irrespective of their acidity. Such information is not fully rooted in scientific data. The exquisite enantioselectivity exhibited by many biologic systems suggests that enantiopurity is an important parameter in any pharmacological effect including pharmacokinetics, metabolic rate, and toxicity. Thus, the racemic components likely interact differentially with biomolecules of the skin. Whether such a concern is of importance for the effects elicited by hydroxyacids is not settled.
The clinical indications of hydroxyacids are multiple. Both AHAs and BHAs exert undisputable direct effects on the stratum corneum (SC), at least in the presence of xerosis, ichthyosis, and analogous conditions. Some hyperpigmentation disorders benefit from the same compounds. Comedonal hyperkeratosis in acne-prone subjects is commonly improved. In the field of benign tumors, keratoses and viral warts are effectively treated by high concentration formulations. Such treatment is largely related to the pH-related chemical burn. Similar caustic effects are also induced in order to perform skin peelings. The effect of hydroxyacids on actinodermatosis appears more complex, involving multifaceted mechanisms, boosting some of the deficient physiologic aspects of aging skin.
Most of the aforementioned effects are in part hydroxyacid dose-dependent. In this chapter, the category low concentration is arbitrarily defined when there is less than 4% of active compound in the formulation. Medium concentration applies to the range 4%–12%, and high concentration for upper dosages. From a regulatory viewpoint, the U.S. Food and Drug Administration (FDA) considers hydroxyacids as cosmetic ingredients provided a maximum 10% concentration, formulated above pH 3.5. Above this concentration and/or below this pH, they fall under the drug categorization.
REFERENCES
1. Piérard GE, Piérard-Franchimont C, Xhauflaire-Uhoda E, Quatresooz P. Remedial cosmetics at the crossroads
between cosmetology and dermatology. Household Pers Care 2007;3:54–5.
2. Saint-Leger D. “Cosmeceuticals”. Of men, science and laws… Int J Cosmet Sci 2012;34:396–401.
3. Rippke F, Schreiner V, Schwanitz HJ. The acidic milieu of the horny layer. New findings on the physiology
and pathophysiology of skin pH. Am J Clin Dermatol 2002;3:261–72.
4. Parra JL, Paye M, the EEMCO Group. EEMCO guidance for the in vivo assessment of skin surface pH.
Skin Pharmacol Appl Skin Physiol 2003;16:188–202.
5. Tung RC, Bergfeld WF, Vidinos AT, Renzi BK. α-Hydroxy acid-based cosmetic procedures. Guidelines
for patient management. Am J Clin Dermatol 2000;1:81–8.
6. Piérard GE, Goffin V, Hermanns-Lê T, Piérard-Franchimont C. Corneocyte desquamation. Int J Mol Med
2000;6:217–21.
7. Piérard-Franchimont C, Piérard GE. Beyond a glimpse at seasonal dry skin. A review. Exog Dermatol
2002;1:3–6.
8. Piérard GE, Masson P, Rodrigues L et al. EEMCO guidance for the assessment of dry skin (xerosis) and
ichthyosis: Evaluation by stratum corneum strippings. Skin Res Technol 1996;2:3–11.
9. Piérard-Franchimont C, Henry F, Piérard GE. The SACD method and the XLRS squamometry tests
revisited. Int J Cosmet Sci 2000;22:437–46.
10. Piérard-Franchimont C, Petit L, Piérard GE. Skin surface patterns of xerotic legs: The flexural and accretive
types. Int J Cosmet Sci 2001;23:121–6.
- Related articles
- Related Qustion
- Uses in Cosmetics of Hydroxyacids Mar 3, 2022
Hydroxyacids are well known for their use in the cosmetics industry. They are often found in products that aid in the reduction of wrinkles, that soften strong, defining lines, and that improve the overall look and feel of the skin.
Vitamin C is possibly the most well-known vitamin in the world. In one of the first controlled experiments in history by a surgeon of the British Royal Navy, James Lind proved in 1747 that giving sailors lime or lemon juice prevented the dr....
Mar 3,2022Biochemical EngineeringNiacinamide, an amide of nicotinic acid (also known as vitamin B3), is a water-soluble vitamin found most commonly in wheat, meat, and fish. Niacinamide acts as an antioxidant but also possesses biological activities, making it an important....
Mar 3,2022API





