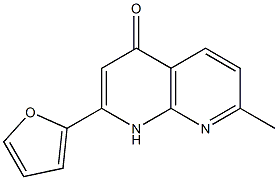
Hemoglobin: Unveiling Allosteric Mechanisms for Enhanced Oxygen Transport
Mar 20,2024
General Description
Hemoglobin, a vital protein in vertebrates, facilitates oxygen transport from lungs to tissues through a cooperative binding mechanism, transitioning between low-affinity tense (T) and high-affinity relaxed (R) states. This allostery is influenced by homotropic effects of oxygen and heterotropic effects from ligands like 2,3-bisphosphoglycerate, modulating oxygen affinity. Structurally, Hemoglobin is a tetramer with each subunit containing a heme group for oxygen binding. Advances in understanding Hemoglobin's allostery, including models like MWC and TTS, highlight its complex regulation through conformational changes. These insights are pivotal for developing tailored Hemoglobin-Based Oxygen Carriers, exploiting the protein's allosteric properties for medical applications.

Figure 1. Hemoglobin
Structure
Hemoglobin is a crucial protein in vertebrates, primarily responsible for transporting oxygen (O2) from the lungs to tissues. It operates through a cooperative mechanism, as depicted by the oxygen equilibrium curve, which shows how Hb's oxygen saturation levels change with varying partial pressures of O2. This process is fundamentally guided by Hemoglobin's ability to switch between two states: the tense (T) state, which has low affinity for O2, and the relaxed (R) state, with high O2 affinity. The transition between these states is influenced by various ligands, including 2,3-bisphosphoglycerate and protons, which modulate Hemoglobin's oxygen affinity and thus its ability to release or bind oxygen efficiently. Structurally, Hemoglobin is a tetramer composed of two alpha (α) and two beta (β) subunits, forming a symmetric arrangement that creates a central water cavity. This cavity varies in size between the T (deoxygenated) and R (oxygenated) states, reflecting the structural changes Hb undergoes upon oxygen binding. Each subunit contains a heme group, where oxygen binds. The heme consists of a ferrous ion surrounded by a porphyrin ring, anchored to the protein via a histidine residue. Oxygen binding induces significant conformational changes across Hemoglobin, affecting not only the heme but also the overall structure of the protein, demonstrating the phenomenon of allostery. Moreover, other molecules like carbon monoxide can bind to Hemoglobin with higher affinity than oxygen, posing toxic risks. These binding events highlight the intricate balance Hemoglobin maintains in facilitating oxygen transport while being susceptible to interference by other ligands, underlining the protein's complex functionality and structural dynamics. 1
Allostery
Hemoglobin allostery, a fascinating aspect of biochemistry, refers to the protein's ability to regulate its function through structural changes induced by the binding of ligands at sites distinct from the active site. This process is governed by two primary allosteric regulations: homotropic effects, which involve the cooperative binding of oxygen molecules, and heterotropic effects, mediated by the binding of various effectors such as protons, CO2, chloride ions, and organic phosphates at non-heme sites. These mechanisms not only facilitate oxygen transport from the lungs to peripheral tissues but also play crucial roles in pH regulation and CO2 scavenging. Historically, the homotropic effects were first conceptualized in models like Linus Pauling's sequential model and later the concerted model by Monod, Wyman, and Changeux (MWC), which proposed a balance between high and low-affinity states of Hemoglobin without requiring quaternary structure changes for cooperativity. This MWC model gained support over time, especially with studies on Hemoglobin encapsulated in silica gels, which allowed detailed observation of Hb's functional states without the constraints of crystal structures. Recent advancements have led to the development of the Tertiary-Two State (TTS) model, which refines the MWC model by suggesting that oxygen and heterotropic effectors influence the equilibrium between low- and high-reactivity tertiary conformations within both quaternary states of Hemoglobin. This model preserves the essence of MWC regarding cooperativity arising from quaternary equilibrium while allowing for a broader range of oxygen affinity across the T and R states. The structural basis for these functional differences has been linked to the "allosteric core" of Hemoglobin, which mediates changes following heme ligation. Despite significant progress in understanding Hemoglobin allostery, complete structural descriptions of all functional states remain elusive, though advances in cryo-electron microscopy hold promise for future discoveries. The insights gained from studying Hemoglobin allostery are crucial for developing Hemoglobin-Based Oxygen Carriers (HBOCs) with tailored properties for medical applications, highlighting the potential to modulate Hb oxygen affinity by adjusting the equilibrium between the tertiary conformations. 2
Reference
1. Ahmed MH, Ghatge MS, Safo MK. Hemoglobin: Structure, Function and Allostery. Subcell Biochem. 2020; 94: 345-382.
2. Faggiano S, Ronda L, Bruno S, et al. From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol Aspects Med. 2022; 84: 101050.
- Related articles
- Related Qustion
- Hemoglobin synthesis and Low effects of hemoglobin Jul 24, 2024
Hemoglobin is a protein containing iron that facilitates the transport of oxygen in red blood cells. Almost all vertebrates contain hemoglobin,with the exception of the fish family Channichthyidae.
- What is a Normal Hemoglobin Level? Dec 28, 2022
A hemoglobin test measures the amount of hemoglobin in the blood. Hemoglobin is a protein in red blood cells that transports oxygen to the organs and tissues of the body and carbon dioxide from them back to the lungs.
HEMOGLOBIN
9008-02-0You may like
- Hemoglobin
-

- $0.00 / 1kg
- 2025-04-11
- CAS:9008-02-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10KG
- Hemoglobin
-

- $0.00 / 1KG
- 2024-10-28
- CAS:9008-02-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 500000kg
- HEMOGLOBIN
-

- $6.60 / 1KG
- 2020-01-03
- CAS:9008-02-0
- Min. Order: 1KG
- Purity: 97%-99%
- Supply Ability: 1kg -1000kg