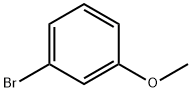
In-Depth Exploration of 3-Bromoanisole and Its Deuterated Analogue
Apr 16,2025
3-Bromoanisole is a useful brominating reagent. It is formed as reaction product in the reaction between HOBr and anisole. Suzuki coupling of 3-bromoanisole with phenylboronic acid catalyzed by palladium pincer complexes has been studied. Heck Reaction of 4-bromoanisole with ethyl acrylates in room-temperature ionic liquids is reported to afford ethyl 3-methoxycinnamate.

General procedure of 3-Bromoanisole
Commercially available 3/4-bromophenol(1 equiv.) was used as a raw material. The corresponding halogenated hydrocarbons (1.5 equiv.), K2CO3 (3 equiv.), and catalyst KI (0.1 equiv.) were sequentially dissolved in acetonitrile solution (15mL), then refluxed at 65 C for 6 h to obtain the crude product, which was subsequently dissolved in a mixture of 1,4-dioxane/H2O (9:1, v/v)mixed solvent (20 mL), then sequentially added (3-(methoxycarbonyl)-5-nitrophenyl)boronic acid (1.5 equiv.), Na2CO3 (3.0 equiv.), Pd(PPh3)4(0.05 equiv.), then reacted at 80 C for 18 h under the N2 atmosphere. The product obtained above was dissolved in a dichloromethane/methanol (1:1, v/v) solution (20 mL) containing 5% Pd/C (10%) and reacted at room temperature for 12 h. The crude product 3-Bromoanisole is separated into different sections and stored away. Condensation with benzoic acid and 3-phenoxybenzoic acid following oxalyl chloride chlorination, respectively. The final product 3-Bromoanisole is obtained(yield: 87%) as white solid.[1]
Synthesis of D4-3-Bromoanisole
At 0°C, D4-3-bromonitrobenzene (CAS.No.81395-19-6, 177 mg, 1 mmol) and Pd-C (40%, 50 mg) were dissolved in 20 mL of deuterated methanol. The mixture was degassed with argon and filled with 10 atm of deuterium. After reacting at room temperature for 24 hours, the catalyst was filtered out. After removing methanol by rotary evaporation, 10 mL of 0.05 M sulfuric acid was added under an ice-salt bath, and an aqueous sodium nitrite solution (69 mg) was added dropwise, and stirred evenly to form a homogeneous solution. The temperature was slowly raised to boiling, and the temperature was lowered to room temperature after 20 minutes. After the reaction conversion was complete as monitored by TLC, the mixture was extracted five times with 200 mL of dichloromethane. The combined organic phases were dried over anhydrous magnesium sulfate, the desiccant was removed by filtration, and the solvent was removed by rotary evaporation. 20 mL of tetrahydrofuran, 168 mg of sodium bicarbonate and 142 mg of methyl iodide were added, and the mixture was reacted at room temperature for 24 hours. Most of the solvent was evaporated, and the mixture was dissolved in dichloromethane, washed with 2% aqueous sodium hydroxide solution and saturated brine, and dried over anhydrous magnesium sulfate. The desiccant was removed from 3-Bromoanisole by filtration and the solvent was removed by rotary evaporation.[2]
Purification by silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) gave 180 mg of a colorless transparent oil containing 3-Bromoanisole, which was dissolved in 50 mL of anhydrous and oxygen-free tetrahydrofuran, 40 mg of magnesium chips was added, and the mixture was degassed with argon and heated to a slight boiling point to initiate the reaction, then cooled to room temperature, and the excess magnesium was filtered out under an argon environment. Then, a solution of 2-(N,N-dimethylaminomethyl)-1-hexanone (148 mg, 0.95 mmol) in tetrahydrofuran was added dropwise. After the addition was completed, the mixture was stirred at room temperature for 2 hours, heated to reflux for 4 hours, the insoluble matter was filtered out, the solvent was evaporated, the mixture containing 3-Bromoanisole was dissolved in dichloromethane, washed with water, saturated ammonium chloride solution, saturated brine, and water, respectively, and then extracted with dichloromethane. The organic phases were combined and dried over anhydrous magnesium sulfate, the desiccant was filtered out, and the solvent was removed by rotary evaporation. The product was purified by silica gel column chromatography (petroleum ether:ethyl acetate=15:1, 5:1, 1:1) to give 120 mg (45%) of light yellow solid D4-3-Bromoanisole
Vibrational frequency analysis on 3-bromoanisole
In this work, the experimental and theoretical spectra of 3-bromoanisole (3-BA) are studied. FT-IR and FT-Raman spectra of title molecule in the liquid phase have been recorded in the region 4000–100 cm−1. The structural and spectroscopic data of the molecule in the ground state have been calculated by using Hartree–Fock and density functional method (LSDA and MPW1PW91) with the 6-31G (d, p) and 6-311G (d, p) basis sets. The vibrational frequencies are calculated and scaled values have been compared with the experimental FT-IR and FT-Raman spectra. The observed and calculated frequencies are found in good agreement. The DFT-LSDA/6-311G (d, p) calculations have been found are more reliable than the ab initio HF/6-31G (d, p) calculations for the vibrational study of 3-bromoanisole. The optimized geometric parameters (bond lengths and bond angles) are compared with experimental values of the molecule. The alteration of vibrational bands due to the substitutions in the base molecule is also investigated from their characteristic region of linked spectrum.[3]
References
[1]Chen, Ya; Yu, Mingyang; Chen, Lianru; Mao, Jianming; Wang, Wenxin; Yang, Zhongcheng; Cao, Zhijun; (…) Zhang, Luyong; Li, Zheng[European Journal of Medicinal Chemistry, 2024, vol. 270, art. no. 116358]
[2]THIRD RES INST MINISTRY PUBLIC SECURITY - CN119330843, 2025, A
[3]Ramalingam S, Periandy S. Spectroscopic investigation, computed IR intensity, Raman activity and vibrational frequency analysis on 3-bromoanisole using HF and DFT (LSDA/MPW1PW91) calculations. Spectrochim Acta A Mol Biomol Spectrosc. 2011 Feb;78(2):835-43.
- Related articles
- Related Qustion
2-chloro-5-cyanopyridine is a crucial compound in pharmaceutical and agrochemical synthesis due to its reactivity and versatility.....
Apr 16,2025Chemical MaterialsDehydroepiandrosterone acetate (DHEA)is involved in various physiological functions and has been studied in diseases and clinical applications.....
Apr 17,2025API3-Bromoanisole
2398-37-0You may like
- 3-Bromoanisole
-

- $1.00 / 1PCS
- 2025-04-18
- CAS:2398-37-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 10 mt
- 3-Bromoanisole
-

- $99.00/ kg
- 2025-04-17
- CAS:2398-37-0
- Min. Order: 0.00100000kg
- Purity: 99%
- Supply Ability: 5000
- 3-Bromoanisole
-

- $999.00/ ton
- 2025-04-17
- CAS:2398-37-0
- Min. Order: 1ton
- Purity: 99%
- Supply Ability: 5000