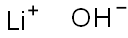Introduction of Lithium hydroxide
Feb 28,2022
General description
Lithium hydroxide, solution appears as a clear to water-white liquid which may have a pungent odor. It is used to make other chemicals. Lithium hydroxide is an inorganic substance with chemical formula of LiOH and English name of lithium hydroxide. It is a white monoclinic fine crystal, spicy, strongly alkaline and corrosive. The pH of 1mol / L solution is about 14, PKB = -0.04. It can absorb carbon dioxide and water in the air, soluble in water, slightly soluble in ethanol and insoluble in ether. The relative density is 1.45, the melting point is 471 ℃ (anhydrous), and it decomposes at 925 ℃. It has two forms: anhydrous and monohydrate Lithium hydroxide is an alkali metal hydroxide. Used for tryptophan determinations in proteins and foods.
Chemical and Physical peoperty
Lithium hydroxide, LiOH, is a light element compound at the crossroads of molecular and ionic bonding. The metastable monomeric molecule is linear (as is LiOLi, but not HOH), yet quite non-rigid. At 298 K (and below) and P=1 atm, LiOH is a hygroscopic ionic solid. Anhydrous LiOH at P=1 atm is relatively stable, melting at 462◦C, attesting to its ionic character. It finds use in tight spaces: as precursor of lithium greases such as lithium stearate,1and as a carbon dioxide absorbant in spacecrafts or submarines (converting CO2to lithium carbonate). Hydrated LiOH exists, as the monohydrate LiOH·H2O, the X-ray and neutron crystal structures of which are available. In the solid state anhydrous LiOH, unlike water ice, for instance, does not feature a hydrogen bonding network; the absence of such bonding in the solid at room temperature is in line with other alkali metal hydroxides (and also amides)[1]. Lithium hydroxide has the universality of alkali, and the following reactions can occur[2]. 1. Alkaline reaction can turn purple litmus test solution blue and colorless phenolphthalein test solution red; The concentrated solution can denature phenolphthalein and change the solution from red to colorless (similar to concentrated NaOH). 2. Neutralize with acid HCl + LiOH = LiCl + H2O 3. React with acid oxide 2lioh + CO2 = Li2CO3 + H2O (this reaction is used to absorb carbon dioxide in Aerospace) 4. React with metal salt solution FeCl3+3LiOH=Fe (OH)3↓+3LiCl.
Figure1 Left: theP4/nmmground-state static structure of LiOH-II at P=1 atm. Red (white, purple) spheres denote O (H, Li) atoms. OH groups and O-Li coordination polyhedra are indicated. Right: coordination polyhedra of OH and Li, respectivel It is easy to absorb carbon dioxide and water in the air, but the absorption capacity is slightly worse than NaOH and KOH.
Application
1.A chelating agent, forming complexes with most metal ions. A ligand Sphingolipids have diverse structural and bioactive functions that play important roles in many key biological processes. Factors such as low relative abundance, varied structures, and a dynamic concentration range provide a difficult analytical challenge for sphingolipid detection. To further improve mass-spectrometry-based sphingolipid analysis, lithium adduct consolidation was implemented to decrease spectral complexity and combine signal intensities, leading to increased specificity and sensitivity. It report the use of lithium hydroxide as a base in a routine hydrolysis procedure in order to effectively remove common ionization suppressants (such as glycolipids and glycerophospholipids) and introduce a source of lithium into the sample. In conjunction, an optimized MALDI matrix system, featuring 2′,4′,6′rihydroxyacetophenone (THAP) is used to facilitate lithium adduct consolidation during the MALDI process. The result is a robust and high-throughput sphingolipid detection scheme, particularly of low-abundance ceramides. Application of our developed workflow includes the detection of differentially expressed liver sphingolipid profiles from a high-fat-induced obesity mouse model. It also demonstrate the method’s effectiveness in detecting various sphingolipids in brain and plasma matrices.
2. The high-pressure phases of crystalline lithium hydroxide, LiOH. Using first-principles calculations, and assisted by evolutionary structure searches, It reproduce the experimentally known phase transition under pressure, but we suggest that the high-pressure phase LiOH-III be assigned to a new hydrogen-bonded tetragonal structure type that is unique amongst alkali hydroxides. LiOH is at the intersection of both ionic and hydrogen bonding, and we examine the various ensuing structural features and their energetic driving mechanisms. At P=17 GPa, we predict another phase transition to a new phase, Pbcm-LiOH-IV , which we find to be stable over a wide pressure range. Eventually, at extremely high pressures of 1100 GPa, the ground state of LiOH is predicted to become a polymeric structure with an unusual graphitic oxygen-hydrogen net. However, because of its ionic character, the anticipated metallization of LiOH is much delayed.
Synthesis
Lithium hydroxide by double decomposition method is usually prepared by mixing lithium carbonate and lime milk into slurry, heating with 100 ℃ steam and filtering out calcium carbonate. This method is commonly used. Li2CO3 (s) + Ca (OH) 2 = 2lioh (s) + CaCO3 ↓ prepared by the reaction of metal lithium and water by redox method. The raw materials of this method are more expensive and less used. 2Li + 2H2O = 2lioh + H2 ↑ lime sintering method[3,4]:
1. Spodumene concentrate (generally containing 6% lithium oxide) is mixed and ground with limestone, sintered at 1150 ~ 1250 ℃ to produce lithium aluminate and calcium silicate, crushed by wet grinding, leached lithium hydroxide with washing solution, precipitated and filtered, evaporated and concentrated, and crystallized to produce lithium hydroxide monohydrate, The chemical equation is: Li2O · AI2O3 · 4SiO2 + 8Cao → Li2O · Al2O3 + 4 [2CaO · SiO2] Li2O · AI2O3 + Ca (OH) 2 → 2LiOH + Cao · AI2O3
2. The industrial lithium hydroxide is dissolved in cold water under stirring, the insoluble matter is filtered, the filtrate is heated and evaporated to crystallization, and then dried after cooling to prepare lithium hydroxide monohydrate reagent.
3. Anhydrous lithium hydroxide can be obtained by drying lithium hydroxide monohydrate in a dryer containing phosphorus pentoxide for several days. It can also be prepared by slowly heating lithium hydroxide monohydrate in hydrogen gas stream to 140 ℃ for dehydration
Storage and Safety
Contact may cause severe irritation to skin, eyes, and mucous membranes. It may be toxic by ingestion, inhalation and skin absorption. Store in a dry and clean warehouse. Keep away from kindling and heat sources. Protect from direct sunlight. Package sealing. It shall be stored separately from oxidants, acids, carbon dioxide and edible chemicals, and mixed storage shall not be allowed. The storage area shall be equipped with appropriate materials to contain leakage. Precautions for transportation during railway transportation, the dangerous goods shall be loaded in strict accordance with the dangerous goods loading table in the rules for the transportation of dangerous goods of the Ministry of railways. The package shall be complete and the loading shall be stable at the time of shipment. During transportation, ensure that the container does not leak, collapse, fall or damage. It is strictly prohibited to load and transport with oxidants, acids, edible chemicals, etc. During transportation, the transport vehicle shall be equipped with leakage emergency treatment equipment. During transportation, it shall be protected from sun exposure, rain and high temperature. During highway transportation, drive according to the specified route, and do not stay in residential areas and densely populated areas. The waste is treated and neutralized in the sewage treatment plant. If possible, reuse containers or bury them in specified places. After measuring for hours, neutralize the aqueous solution of the product, filter out the solid for burial, and flush the solution into the sewer. The reaction produces heat and smoke, which is controlled by controlling the addition rate.
Reference
1.Hermann A., Ashcroft N. W. & Hoffmann R., "Lithium hydroxide, LiOH, at elevated densities," The Journal of Chemical Physics, Vol.141, No.2(2014), p.24505.
2,Tran A., Wan L. & Xu Z. et al., "Lithium Hydroxide Hydrolysis Combined with MALDI TOF Mass Spectrometry for Rapid Sphingolipid Detection," Journal of the American Society for Mass Spectrometry, Vol.32, No.1(2021), pp.289-300.
3.Deng shunjiao, sun Hongbo, Qin Jiazheng, etc.: Research Progress of lithium hydroxide preparation process, salt lake research, 2019, No. 04, pp. 77-81.
4. Patent Application Publication (10) Pub. No.: US 2011/0044882A1
- Related articles
- Related Qustion
Creatine monohydrate, also known as N-methylguanidinoacetic acid, inosine, forminylacetic acid, and creatine sarcosine, is a pharmaceutical raw material and an additive for health care products.....
Feb 28,2022APIPotassium hydroxide is an inorganic compound which is denoted by the chemical formula KOH. Potassium hydroxide is also known as caustic potash, lye, and potash lye.....
Feb 28,2022Inorganic saltsLithium hydroxide
1310-65-2You may like
Lithium hydroxide manufacturers
- Lithium Hydroxide
-

- $1.00 / 1PCS
- 2025-11-14
- CAS:1310-65-2
- Min. Order: 1PCS
- Purity: 99%
- Supply Ability: 10 mt
- Lithium hydroxide
-

- $35.00 / 1kg
- 2025-09-26
- CAS:1310-65-2
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 600tons
- Lithium hydroxide
-

- $10.00 / 1kg
- 2025-09-26
- CAS:1310-65-2
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 300tons