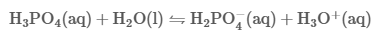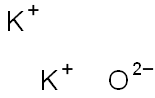Is k2o ionic or covalent?
Mar 15,2024
Potassium oxide(K2O)also called Potassium Monoxide, Di-potassium Hydroxide, and Kalium Oxide, is an ionic compound formed by combining potassium and oxygen.
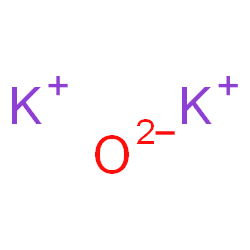
Potassium cannot be found in its natural state because it is highly reactive. It has valency +1 and combines readily with oxygen atoms forming K2O. When potassium is oxidized, Potassium Oxide, K2O, is formed as a grey crystalline substance. Potassium oxide is a strongly corrosive alkali, when dissolved in water.
Potassium Oxide is widely used as a fertilizer in agriculture.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringPhosphoric acid, also known as orthophosphoric acid or phosphoric(V) acid, is a mineral (inorganic) acid having the chemical formula H3PO4. The pH of a 0.15 M solution of phosphoric acid (H3PO4) is measured to be 1.54.....
Mar 15,2024Inorganic chemistryPotassium oxide
12136-45-7You may like