Itraconazole:Sporanoxs
Mar 28,2022
DESCRIPTION
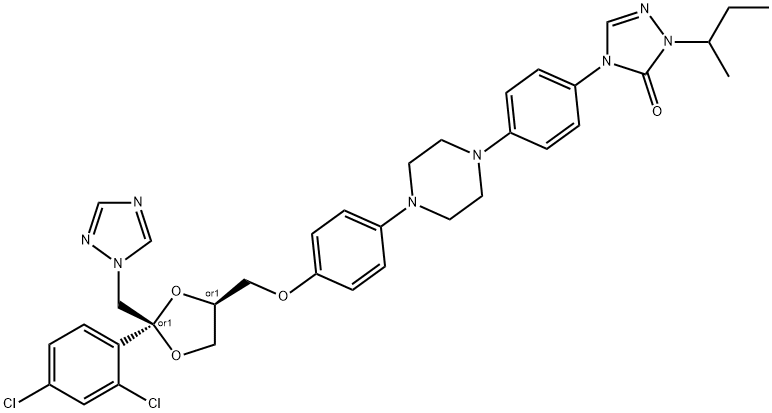
Itraconazole (Sporanoxs) is a synthetic triazole drug developed in the 1980s as the first orally bioavailable triazole agent. Similar to posaconazole, it contains a piperazine-phenyl-triazole side chain. Available preparations include a 100-mg capsule and an oral solution (10 mg/ml complexed with 400 mg/ml hydroxypropyl-b-cyclodextrin). Although not currently available, itraconazole has also been formulated for intravenous administration. Itraconazole was the first orally active agent against aspergillosis and sporotrichosis and currently has US Food and Drug Administration (FDA) approval for the treatment of aspergillosis, blastomycosis, histoplasmosis, mucosal candidiasis, febrile neutropenia, and onychomycosis. Itraconazole has the chemical name (7)-1-[(RS)-sec-butyl]-4-[p-[4-[p-[[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]- phenyl]-1-piperazinyl]phenyl]-D2-1,2,4-triazolin-5-one and is a racemic mixture of four diastereomers (two enantiomeric pairs).
MECHANISM OF DRUG ACTION
The mechanism of action for itraconazole is similar to the other triazole antifungal agents and involves inhibition of cytochrome P450 upon binding of the triazole ring. In the presence of itraconazole, methylated sterol precursors accumulate in the fungal cell membrane, thereby increasing membrane permeability and altering membrane-bound enzyme function, ultimately halting cell division and leading to cell death. Itraconazole has a high affinity for fungal P450 cytochrome enzymes and binds more weakly to mammalian cytochrome P450 enzymes.
Drug interactions
Itraconazole is both a substrate and an inhibitor of the CYP450 enzymes, specifically CYP3A4 and CYP2C9. Studies have identified numerous interacting drugs that either reduced the clearance of the co-administered drug or itraconazole, or enhanced the metabolism of itraconazole. These interactions often required therapeutic drug monitoring and dose adjustment or occasionally avoidance of the drug due to the magnitude and clinical relevance of the interaction. A thorough review of a patient’s medications should be examined for potential drug interactions before start and end of therapy.
Itraconazole is a more potent inhibitor of CYP3A4 than fluconazole, voriconazole, and posaconazole. Co-administration of itraconazole with cisapride, dofetilide, pimozide, terfenadine, levacetylmethadol, or quinidine results in elevated concentrations of these drugs and is contraindicated owing to the risk of QT prolongation, torsades de pointes, and ventricular arrhythmia. Lovastatin, simvastatin, oral midazolam, triazolam, and ergot alkaloid clearance are also markedly reduced and co-administration is contraindicated. Itraconazole interferes with the metabolism of midazolam, resulting in a 6- to 7-fold increase in AUC, a 2.5-fold increase in peak serum concentrations, and excess sedation due to prolonged midazolam exposure in healthy volunteers.
Co-administration of oral midazolam and itraconazole is contraindicated and it is recommended that intravenous midazolam be used cautiously. The intravenous midazolam dosage should be reduced substantially and patients should be monitored for signs of toxicity. Likewise, co-administration with triazolam is contraindicated and use with other benzodiazepines, including diazepam and alprazolam, is not recommended. Itraconazole may expose patients to higher levels of vinca alkaloids. Concomitant use of itraconazole and vincristine in patients with leukemia or lymphoma was associated with increased risk of neurotoxicity, including severe myalgias, arthralgias, paralysis, and paralytic ileus. These symptoms were generally reversible with discontinuation of itraconazole. Patients receiving both itraconazole and a vinca alkaloid should be monitored closely for signs of neurotoxicity.
- Related articles
- Related Qustion
- What is itraconazole used to treat? Mar 16, 2024
Itraconazole is a triazole antimycotic itraconazole used to treat systemic infections such as candidosis and aspergillosis including disseminated infections, blastomycosis, coccidioidomycosis, histoplasmosis, and cryptococcosis.
Fluconazole is currently approved by the US Food and Drug Administration (FDA) for treatment of cryptococcosis and candidiasis, including invasive, oroesophageal, urogenital, and vulvovaginal disease.....
Mar 28,2022APISimilar to rifampicin, rifabutin (also known as ansamycin LM 427) is a derivative of rifamycin S. The most important property of this drug is that it is more active than rifampicin against Mycobacterium avium complex (MAC) in vitro, against....
Mar 28,2022APIItraconazole
84625-61-6You may like
- Itraconazole
-

- 2025-12-01
- CAS:84625-61-6
- Min. Order:
- Purity: 0.99
- Supply Ability:
- Itraconazole
-

- $0.00 / 1Kg/Bag
- 2025-12-01
- CAS:84625-61-6
- Min. Order: 1KG
- Purity: 98.5%-101.5%
- Supply Ability: 200KG
- Itraconazole
-
- $100.00 / 25KG
- 2025-11-27
- CAS:84625-61-6
- Min. Order: 1KG
- Purity: 99%min
- Supply Ability: 200TON