Linoleic Acid: Role in Human Diet and Food Sources
Jun 5,2024
General Description
Linoleic acid, a prevalent PUFA in the modern diet, is primarily found in vegetable oils, nuts, seeds, meats, eggs, and processed foods, with soybean oil contributing significantly in the US. As a crucial energy source, it is also vital in the formation of lipids and cell signaling compounds. While it can be converted to arachidonic acid, which yields bioactive compounds, excessive production is linked to chronic diseases. Tracer studies show a low conversion rate from linoleic acid to arachidonic acid, challenging the notion that reducing linoleic acid intake limits tissue levels of arachidonic acid. After absorption, its fate in tissues is determined by their specific needs.

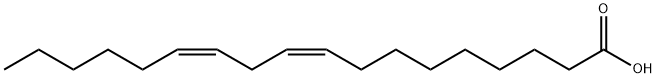
Figure 1. Linoleic acid
Food Sources
Linoleic acid is predominantly found in various food sources, including vegetable oils, nuts, seeds, meats, and eggs. In the United States, the consumption of linoleic acid began to surge around 1969, coinciding with the widespread use of soybean oil as a primary additive in numerous processed foods. As a result, manufactured foods containing soybean oil are notably abundant in linoleic acid. Currently, soybean oil contributes to approximately 45% of the dietary intake of linoleic acid in the US. Moreover, linoleic acid stands as the most prevalent polyunsaturated fatty acid (PUFA) in a wide array of food items. Despite accounting for roughly 88% of the total PUFAs in soybean oil, its levels exceed 70% in most commonly consumed foods. For instance, linoleic acid comprises between 70 and 85% of all PUFAs in meats such as beef, chicken, and pork, and exceeds 80% in eggs. Even low-fat foods like vegetables, fruits, and grains are notably rich in linoleic acid as the primary PUFA, with the exception of beans, where linoleic acid constitutes between 40 and 50% of the total PUFAs. It is worth noting that most vegetable oils are linoleic acid-based, with flaxseed being a notable exception. Therefore, linoleic acid is pervasive in the modern diet, reflecting its wide distribution across a broad range of commonly consumed foods. 1
Role in Human Diet
Linoleic acid, also known as 18:2ω6 or cis, cis-9,12-octadecadienoic acid, stands as the most prevalent polyunsaturated fatty acid (PUFA) consumed in the human diet. Upon ingestion, linoleic acid undergoes four primary fates. Firstly, akin to all fatty acids, it serves as a crucial energy source within the body. Secondly, it can be esterified to generate neutral and polar lipids, including phospholipids, triacylglycerols, and cholesterol esters. Notably, within membrane phospholipids, linoleic acid acts as a structural component, crucial for maintaining the requisite membrane fluidity of the epidermis' transdermal water barrier. Furthermore, upon release from membrane phospholipids, linoleic acid is subject to enzymatic oxidation, producing various derivatives essential for cell signaling, such as 13-hydroxy or 13-hydroperoxy octadecadienoic acid (13-H(P)ODE). As the foundational compound for the family of ω6 PUFAs, linoleic acid can be elongated and desaturated, leading to the formation of other bioactive ω6 PUFAs, including γ-linolenic acid (18:3ω6) and arachidonic acid (20:4ω6). Subsequently, arachidonic acid can be converted into a myriad of bioactive compounds known as eicosanoids, such as prostaglandins and leukotrienes, which play pivotal roles in normal cellular and tissue metabolic functions. However, excessive production of these eicosanoids is associated with chronic diseases like inflammation and cancer. Notably, linoleic acid has gained notoriety due to its potential conversion to arachidonic acid. Despite hypotheses suggesting that restricting linoleic acid intake could reduce tissue levels of arachidonic acid, this does not seem to hold true for individuals consuming a typical Western diet. Tracer kinetic studies indicate that the fractional conversion of linoleic acid to arachidonic acid is estimated to be between 0.3% and 0.6%, with this conversion appearing to be offset by turnover. After absorption by enterocytes in the small intestines, linoleic acid is packaged into chylomicrons and enters the general circulation via the thoracic duct. From there, it is delivered to hepatic and extrahepatic tissues, where its fate, whether incorporation into membrane phospholipids or undergoing desaturation and elongation, is determined by the specific needs of the tissue. 2
Reference
1. Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011; 93(5): 950-962.
2. Whelan J, Fritsche K. Linoleic acid. Adv Nutr. 2013; 4(3): 311-312.
- Related articles
- Related Qustion
- Microbial metabolite of linoleic acid ameliorates intestinal inflammation Oct 17, 2019
A Japanese research group has demonstrated that 10-hydroxy-cis-12-octadecenoic acid (HYA), a gut microbial metabolite of linoleic acid, has a suppressive effect on intestinal inflammation. HYA is expected to be practically applied as a func
- Effects of linoleic acid on inflammatory response depend on genes Oct 17, 2019
The effects of linoleic acid on the human body are largely dependent on genes, a new study from the University of Eastern Finland shows. Linoleic acid is an essential fatty acid. People carrying different variants of the FADS1 gene had a di
Months ~ years or permanently. Entecavir (ETV) is an oral prescription antiviral medication used to treat liver infections caused by the hepatitis B virus.....
Jun 5,2024DrugsTea Polyphenol exhibits potent antifungal and antibacterial activities by disrupting cell membranes and inducing enzyme activities, making it an effective natural solution for plant diseases.....
Jun 5,2024APILinoleic acid
60-33-3You may like
- Linoleic acid
-

- $46.00/ kg
- 2024-05-13
- CAS:60-33-3
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000kg/week
- Linoleic acid
-

- $135.00 / 1kg
- 2024-03-29
- CAS:60-33-3
- Min. Order: 1kg
- Purity: 94.9%
- Supply Ability: 10 TONS
- Linoleic acid
-
- $5.00 / 1KG
- 2024-03-27
- CAS:60-33-3
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: g-kg-tons, free sample is available