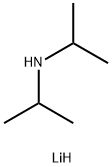
Lithium diisopropylamide-LDA
Jul 5,2022
Lithium diisopropylamide (LDA) is a strong, non-nucleophilic base that is widely used. Because of its reactivity with many solvents, it has historically been generated in the laboratory by reaction of n-butyllithium and diisopropyl amine. Recently, LDA has become commercially available as a solution in THF/heptane/ethyl benzene. The commercially available material is frequently colored, making titration difficult.
Freshly prepared LDA has varying stability, being most stable in alkanes and 1:1 alkanes:THF. Homemade LDA should be stored cold to extend its shelf-life. The preparation of LDA is representative of other lithium amide bases, such as lithium tetramethylpiperidide and lithium hexamethyl disilylamide.
To a solution of diisopropylamine (3.44 g, 4.76 ml, 0.0341 mole) in THF (25 mL) at −78 ºC (methanol–dry ice bath) is added a solution of n-butyllithium (1.61 M in hexane, 21.1 mL, 0.0340 mol) with stirring under argon. The solution is warmed to 0 ºC in 15 min to provide an approximately 0.7 M solution of LDA.
Reference: Enders, D.; Pieter, R.; Renger, B.; Seebach, D. Org. Synth. 1978, 58, 113 or Enders, D.; Pieter, R.; Renger, B.; Seebach, D. Org. Synth. 1988, Coll. Vol. 6, 542.
- Related articles
- Related Qustion
- What is Lithium diisopropylamide? Feb 22, 2021
Lithium diisopropylamide (commonly abbreviated LDA) is a chemical compound with the molecular formula [(CH3)2CH]2NLi. It is used as a strong base and has been widely utilized due to its good solubility in non-polar organic solvents.
Pregabalin is a 3-isobutyl derivative of gamma-amino butyric acid (GABA) with anti-convulsant, anti-epileptic, anxiolytic, and analgesic activities....
Jul 5,2022APIThe essential trace mineral, selenium, is of fundamental importance to human health. As a constituent of selenoproteins, selenium has structural and enzymic roles, in the latter context being best-kno....
Jul 6,2022APILithium diisopropylamide
4111-54-0You may like
Lithium diisopropylamide manufacturers
- Lithium diisopropylamide
-

- $10.00 / 1ASSAYS
- 2025-12-15
- CAS:4111-54-0
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 1 ton
- Lithium diisopropylamide
-

- $10.00 / 1KG
- 2025-12-11
- CAS:4111-54-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 100 mt
- Lithium diisopropylamide
-

- $10.70 / 1kg
- 2025-05-26
- CAS:4111-54-0
- Min. Order: 10kg
- Purity: 99%
- Supply Ability: 10000kg