Mechanism of action of Nikkomycin Z
Mar 31,2022
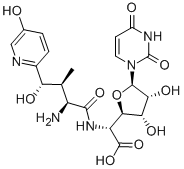
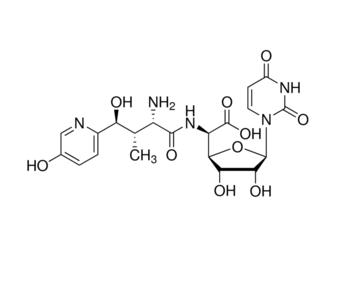
Nikkomycin Z is an experimental compound with antifungal properties. Like all nikkomycins, it consists of a pyrimidine nucleoside linked to a short peptide moiety. There are more than 14 naturally occurring nikkomycins and a number of synthetic analogs. Only the nikkomycin Z analog has shown clinically useful antifungal activity in vitro as well as in animal models.
Uses
Despite extensive evaluation of nikkomycins for commercial development, no product has reached the marketplace. This is most likely due to the absence of clinically useful in vivo activity, a result of competition of endogenous peptides for dipeptide permease. Other possible reasons include degradation by various peptidases and the differential susceptibilities of the target isoenzyme(s) to the inhibitory effects of nikkomycins.
Despite these drawbacks, nikkomycin Z is undergoing further clinical development for use in treatment of coccidioidomycosis. This compound is also a candidate for further study as an agent against other endemic fungi in combination with cell wall-active agents or triazoles.
Mechanism of action
Nikkomycin Z is a potent competitive inhibitor of chitin synthase which leads to fungal growth inhibition and cell death. Its biological activity relies on its structural similarity to UDP-GlcNAc, the substrate for chitin biosynthesis. The susceptibility of an organism to nikkomycin Z depends on the chitin content as well as the differences in distribution and function of each of the chitin synthase isoenzymes. In addition, given that the active site of the enzyme is situated on the inner face of the fungal plasma membrane, the effectiveness of the compound is dependent on its ability to enter the cell; this process is mediated via a dipeptide permease-mediated peptide-uptake transport system. Finally, antifungal activity is dependent on the affinity of nikkomycin Z for each chitin synthase isoenzyme since the active site domains of the various isoenzymes are different.
Toxicity
Since chitin is absent in mammalian cells, toxicity is anticipated to be low. In animal models of endemic mycoses, the drug was well tolerated. No toxicity was demonstrated in mice at doses of up to 400 mg/kg body weight per day for 28 days.
- Related articles
- Related Qustion
Haloprogin (3-iodo-2-propynyl 2,4,5-trichlorophenyl ether; Halotex cream) has been used as a topical agent for the treatment of dermatophyte infections. Haloprogin was synthesized in 1963 by Seki as one of a series of halophenol-g-iodopropa....
Mar 31,2022APIMethyl orange is an organic substance with the chemical formula of C14H14N3SO3Na, which is often used as an acid-base indicator.....
Mar 31,2022API