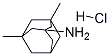
Memantine HCl: Pharmacokinetics and Therapeutic Efficacy
Dec 16,2024
Memantine hydrochloride, a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist, is used to treat Alzheimer’s disease (AD). As AD is a progressive neurodegenerative disorder, achievement of steady drug concentration and patient compliance may be essential to maintain treatment effectiveness. A new dry syrup formulation containing memantine hydrochloride has been developed to improve medication adherence in AD patients and to reduce family and caregiver burden.

Pharmacokinetics
Memantine is well absorbed after oral administration and has linear pharmacokinetics over the therapeutic dose range. It is excreted predominantly in the urine, unchanged, and has a terminal elimination half life of about 60-80 hours.
The mentioned pharmacokinetic characteristics of memantine transdermal patch in animal may be considered in human use as follows. The longer duration and lower peak concentration with same drug exposure as compared to oral administration can provide fewer dosing rate and prevent adverse event related to peak drug concentration after administration, respectively.
The new dry syrup formulation containing memantine hydrochloride showed bioequivalence to the film-coated tablet under the two dosing conditions. Thus, the new dry syrup is suitable under either dosing condition for patients with AD.
Therapeutic Efficacy
Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 25 million cases worldwide. Until recently, the pharmacotherapy of AD was limited to the use of cholinesterase inhibitors (ChEIs) that are approved only for the mild to moderate stages of the illness.
More recent studies have examined the role of memantine in the treatment of the mild to moderate stages of the disease, although the collective results of these studies remain inconclusive. Available pharmacoeconomic data indicate that treatment with memantine is cost-effective when compared with no treatment in patients with moderate to severe AD. Memantine treatment is predicted to be associated with lower costs of care, longer time to dependence and institutionalization, and gains in quality-adjusted life-years.
References:
[1] RAJESH R TAMPI Christopher H van D. Memantine: efficacy and safety in mild-to-severe Alzheimer’s disease.[J]. Neuropsychiatric Disease and Treatment, 2007, 3 2. DOI:10.2147/nedt.2007.3.2.245.
[2] SOO-HAN LEE. Pharmacokinetics of Memantine after a Single and Multiple Dose of Oral and Patch Administration in Rats[J]. Basic & Clinical Pharmacology & Toxicology, 2015, 118 2: 97-170. DOI:10.1111/bcpt.12479.
[3] YUTARO MAEKAWA. Pharmacokinetics and Bioequivalence of Memantine Tablet and a New Dry Syrup Formulation in Healthy Japanese Males: Two Single-Dose Crossover Studies[J]. Advances in Therapy, 2019, 36 10. DOI:10.1007/s12325-019-01044-y.
- Related articles
- Related Qustion
Supplementation with pyridoxal 5'-phosphate monohydrate can synthesize neurotransmitters such as dopamine and serotonin, maintaining a healthy nervous system.....
Nov 4,2025Biochemical EngineeringKetoprofen, chemistry 3-benzoyl by name-Alpha-Methyl toluylic acid, is a non-steroidal anti-inflammatory analgesics, has anti-inflammatory, analgesic, analgesic effects.....
Jul 11,2024APIMemantine HCl
41100-52-1You may like
- Memantine HCl
-

- $1.00 / 1g
- 2025-12-20
- CAS:41100-52-1
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Memantine HCl
-

- $0.00 / 1KG
- 2025-12-19
- CAS:41100-52-1
- Min. Order: 1KG
- Purity: 99.0~101.0%
- Supply Ability: 500KG/month
- Memantine hydrochloride
-

- $50.00 / 500mg
- 2025-12-17
- CAS:41100-52-1
- Min. Order:
- Purity: 99.96%
- Supply Ability: 10g