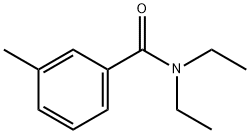
N,N-Diethyl-3-methylbenzamide (DEET): Synthesis, Composition, Applications, and Storage Guidelines
Dec 13,2024
N,N-Diethyl-3-methylbenzamide(DEET) is the oldest, one of the most effective, and most common active ingredients in commercial insect repellents. It is a slightly yellow oil intended to be applied to the skin or to clothing and provides protection against mosquitoes, flies, ticks, fleas, chiggers, leeches, and many other biting insects.

Applications
N,N-Diethyl-3-methylbenzamide(DEET) is effective against a variety of invertebrates, including ticks, flies, mosquitos, and some parasitic worms. DEET is the key active ingredient in many commercial mosquito-repellent formulations, and is also an environment-friendly compound, which well matches the biodegradability and compostability of PLLA. PLLA/DEET blends at various compositions were prepared via melt extrusion.1
Synthesis
N,N-Diethyl-3-methylbenzamide is a slightly yellow liquid at room temperature, it can be prepared by converting m-toluic acid (3-methylbenzoic acid) to the corresponding acyl chloride using thionyl chloride (SOCl2), and then allowing that product to react with diethylamine:
Storage Methods
N,N-Diethyl-3-methylbenzamide(DEET) should be stored in a cool, dry place away from direct sunlight and heat sources. Containers should be tightly sealed to prevent exposure to air and moisture, which can degrade the compound. Additionally, it should be kept separate from oxidizing agents and acids to prevent unwanted reactions that could compromise its integrity.
References:
[1] MARIA LAURA DI LORENZO Alessandra L. N,N-Diethyl-3-methylbenzamide (DEET): A mosquito repellent as functional plasticizer for poly(l-lactic acid)[J]. Thermochimica Acta, 2019, 677: 1-216. DOI:10.1016/j.tca.2019.02.004.
- Related articles
- Related Qustion
- Toxicity of DEET Jan 14, 2022
DEET was first developed and patented by the US Army in 1946. It was approved for general public use by the US Environmental Protection Agency (EPA) in 1957 and was reregistered in 1998. It has been estimated that more than 1.8 million kg (
N,N-Diethyl-m-toluamide
134-62-3You may like
N,N-Diethyl-m-toluamide manufacturers
- Cardiogen 20mg (Bioregulator)
-

- $110.00/ kit
- 2025-10-25
- CAS:134-62-3
- Min. Order: 1kit
- Purity: 99%
- Supply Ability: 10000kits
- N,N-Diethyl-m-toluamide
-
- 2025-10-25
- CAS:134-62-3
- Min. Order:
- Purity: 0.99
- Supply Ability:
- N,N-Diethyl-m-toluamide
-

- $0.00 / 25KG
- 2025-10-24
- CAS:134-62-3
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month