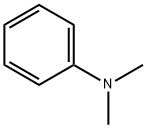
N,N-Dimethylaniline: Synthesis, applications and toxicity
May 30,2023
General description
N,N-Dimethylaniline (DMA) belongs to the N-dialkylaminoaromatics, a chemical class structurally alerting to DNA reactivity. Its application may be industrial (dye and pesticide intermediates, polymerizing agents) and surgical (polymerization accelerators for the manufacture of bone cements and prosthetic devices), thus implying heterogeneous types of human exposure. Findings of carcinogenicity in rodents and some nonexhaustive genotoxicity data are available for N,N-Dimethylaniline. Its appearance is as follows:

Figure 1 Appearance of N,N-Dimethylaniline
Synthesis
A flask was charged with primary amine (10 mmol), paraformaldehyde (20 mmol) and oxalic acid dihydrate (0.1 mol) and briefly flushed with nitrogen. In the case of secondary amines, 10 mmol of paraformaldehyde and 50 mmol of oxalic acid dihydrate were used. For amines with multiple amino functions, 10 mmol of paraformaldehyde and 50 mmol of oxalic acid dihydrate were applied per methyl group to be introduced. The vessel was closed and heated to 100°C for 1 h. and to 120°C for 10 min. The reaction mixture was cooled to room temperature. The white, crystalline mass obtained was crushed, and calcium oxide (0.1 mol) suspended in 50 ml of ethanol was added. The mixture was stirred vigorously for 30 min, solids were removed by filtration, and the solvent was removed in vacuo to produce the pure amine. For volatile amines, the crystalline reaction product was crushed and dissolved in water (100 ml). The solution was adjusted to a pH of 10 by 10% aqueous sodium hydroxide and twice extracted with ethyl acetate (10 ml). The combined organic phases were dried over Na2SO4, the desiccant was removed, and a 2 M solution of hydrogen chloride in ethyl ether (8 ml) was added. The resulting precipitate, the corresponding aminium hydrochloride, was separated by filtration, washed with ethyl acetate (5 ml) and liberated from residual solvent in vacuo. N,N-Dimethylaniline. M = 121.18 g/mol, yield: 1.22 g (100%). 1H NMR: δ 2.93 (s, 6H, N-(CH3)2), 6.63 (m, 3H, ArCH), 7.24 (m, 2H, ArCH).13C NMR: δ 41.0 (N-CH3), 113.1 (C2, C6), 117.1 (C4), 129.5 (C3, C5), 151.1 (C1) [1].
Applications
In terms of it utilization, N,N-Dimethylaniline finds an indirect use (as intermediates in dye and pesticide synthesis) and a direct use (as analytical reagents and polymerization activators). In this latter use, the N,N-Dimethylaniline find application in curing acrylic resins, which are the most widely employed polymers in dental and orthopedic practice, such as in prosthetic device manufacture and bone cement preparation [2].
Toxicity
To investigate their mechanism of action and mutagenic/ carcinogenic potential, N,N-Dimethylaniline was analyzed for complementary genotoxicity endpoints, namely, gene mutation in Salmonella (Ames test), structural and numerical chromosome aberrations in hamster V79 cells (micronucleus test, matched with an immunofluorescent staining for kinetochore proteins), and in vivo DNA damage in mouse and rat liver (alkaline DNA elution test). The results essentially indicate that both chemicals are chromosome damaging agents. Indeed, at the maximum nontoxic doses, they proved nonmutagenic in Salmonella (although their toxicity did not allow concentrations > 70 kg/plate to be tested) and weakly positive in inducing DNA damage (increases in DNA elution rates at most -2.4 times control value). Conversely, they proved clearly positive in inducing numerical chromosome alterations, with dose-dependent increases up to more than five times the control value for N,N-Dimethylaniline. At the highest dose tested, both chemicals also showed a significant clastogenic effect [3]. Groups of F344 rats and B6C3F1 mice (10/sex/species) were treated by gavage with N,N-Dimethylaniline in corn oil at 0, 31.25, 62.5, 125, 250, or 500 mg/kg/day, 5 days/week for 13 weeks. There were no effects on mortality. Decreased body weight gain occurred in male rats at greater than or equal to 250 mg/kg/day (or higher), but not in other groups. Clinical signs of toxicity included increased salivation after dosing in all treated groups, a dose-related increased incidence of excessive urination in female rats at greater than or equal to 31.25 mg/kg/day, blanching or cyanosis (or both) in all groups of treated animals at greater than or equal to 125 mg/kg/day, and changes in motor activities at all doses in male mice and at greater than or equal to 125 mg/kg/day in all other groups [4].
References
[1]Rosenau et al. A solvent-free and formalin-free Eschweiler-Clarke methylation of amines. Synthetic Communications (2002), 32(3), 457-465.
[2]Brauer GM (1981): Initiator-accelerator systems for acrylic resins and composites. In Gebelein CG, Koblitz FF (eds): Biomedical and dental applications of polymers. “Polymer Science and Technology,’’ Vol 14. New York: Plenum Press, pp 395409.
[3]Taningher M, Pasquini R, Bonatti S. Genotoxicity analysis of N, N‐dimethylaniline and N, N‐dimethyl‐p‐toluidine[J]. Environmental and molecular mutagenesis, 1993, 21(4): 349-356.
[4]Abdo K, Wolfe M, Hiles R. Subchronic toxicity of N, N-dimethylaniline to F344 rats and B6C3F1 mice[C]//Federation Proceedings. 1984, 43: 1698.
- Related articles
- Related Qustion
- N,N-Dimethylaniline: Properties, Analysis and Application Apr 25, 2024
N,N-Dimethylaniline is a tertiary amine used as an intermediate in the manufacture of vanillin, mirex, methyl violet and other dyes, and as a solvent, alkylating agent and stabiliser.
- What is N,N-Dimethylaniline? Aug 20, 2020
N,N-Dimethylaniline is used as chemical intermediate in the manufacturing of vanillin, Michler's ketone, and dyes such as Acid Red 2, Basic Green 4 and Basic Violet 1.
Apigenin is a naturally occurring flavone that belongs to the class of compounds called flavonoids. It is a promising natural compound with numerous potential health benefits.....
May 30,2023APILevamisole hydrochloride has been widely used in treatment of worm infestations in both humans and animals. As an anthelmintic, it probably works by targeting the nematode nicotinergic receptor.....
May 30,2023DrugsN,N-Dimethylaniline
121-69-7You may like
N,N-Dimethylaniline manufacturers
- N,N-Dimethylaniline
-

- $1.00 / 200KG
- 2024-08-27
- CAS:121-69-7
- Min. Order: 1KG
- Purity: ≥99%
- Supply Ability: 2000mt/year
- N,N-Dimethylaniline
-

- $15.00 / 1KG
- 2024-08-20
- CAS:121-69-7
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 50 ton
- N,N-Dimethylaniline
-

- $3.00 / 1mL
- 2024-01-08
- CAS:121-69-7
- Min. Order: 1mL
- Purity: 99%
- Supply Ability: 50000tons