Orlistat: A Comprehensive Overview for Chemical Professionals
Jun 27,2024
Introduction
Orlistat, a potent lipase inhibitor, has become a significant player in the fight against obesity. Marketed under various brand names including Xenical and Alli, Orlistat's primary function is to prevent the absorption of dietary fats in the human digestive system. Given its unique mechanism of action and clinical effectiveness, Orlistat has garnered substantial interest within the pharmaceutical and chemical research communities. This article aims to provide an in-depth analysis of Orlistat, covering its properties, main components, applications, and storage methods.

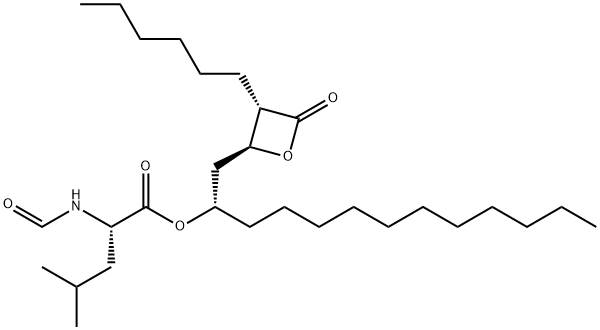
Figure 1 Characteristics of Orlistat
Properties of Orlistat
Orlistat is a white to off-white crystalline powder, characterized by its hydrophobic nature and poor solubility in water. Its chemical formula is C29H53NO5, with a molecular weight of 495.7 g/mol. The compound has a melting point ranging from 42°C to 45°C. Orlistat's structure includes a lactone ring, which is crucial for its biological activity as a lipase inhibitor.
The pharmacokinetic profile of Orlistat is notable for its minimal systemic absorption. Studies indicate that less than 1% of an administered dose is absorbed into the bloodstream, which largely confines its action to the gastrointestinal tract. This limited absorption profile reduces the risk of systemic side effects, making Orlistat a relatively safe option for long-term obesity management.
Main Components
The primary active component of Orlistat is the lipase inhibitor itself, which is chemically synthesized through a multi-step process involving esterification and selective hydrogenation. The synthesis begins with the formation of an intermediate lactone, followed by the introduction of a lipophilic side chain to enhance the molecule's affinity for gastrointestinal lipases.
Inactive ingredients commonly found in Orlistat formulations include microcrystalline cellulose, sodium starch glycolate, and povidone, which aid in the drug's stability and bioavailability. Additionally, the capsule form of Orlistat may contain gelatin, titanium dioxide, and edible ink for branding purposes.
Applications
Orlistat's primary application is in the treatment of obesity, where it serves as an adjunct to lifestyle modifications such as diet and exercise. By inhibiting pancreatic and gastric lipases, Orlistat prevents the hydrolysis of triglycerides into absorbable free fatty acids and monoglycerides. This mechanism results in approximately 30% of ingested fats being excreted undigested, leading to a caloric deficit and subsequent weight loss.
Beyond its primary indication, Orlistat has been investigated for potential benefits in managing other health conditions related to obesity. These include type 2 diabetes, where weight loss facilitated by Orlistat can improve glycemic control, and hyperlipidemia, where the reduction in dietary fat absorption can lower serum cholesterol levels. Preliminary research also suggests possible applications in reducing the risk of certain cancers associated with obesity, such as colorectal cancer.
Storage Methods
Proper storage of Orlistat is crucial to maintain its chemical stability and therapeutic efficacy. Orlistat should be stored at controlled room temperature, ideally between 15°C and 30°C (59°F and 86°F). The drug should be kept in a tightly sealed container to protect it from moisture and light, which can degrade the active compound.
For laboratory and pharmaceutical settings, Orlistat should be handled with care to avoid contamination and degradation. It is recommended to store Orlistat in a dry environment, away from reactive chemicals and substances that could potentially cause hydrolysis of the lactone ring. When handling bulk quantities, personal protective equipment such as gloves and safety goggles should be used to minimize exposure and ensure safe handling practices.
Conclusion
Orlistat represents a significant advancement in the pharmacological management of obesity, offering a targeted approach to reducing dietary fat absorption. Its unique properties, coupled with a favorable safety profile, have solidified its role in obesity treatment regimens. For professionals in the chemical field, understanding the detailed properties, synthesis, and storage requirements of Orlistat is essential for advancing research and optimizing its clinical applications. As obesity continues to pose a major public health challenge, Orlistat remains a valuable tool in the ongoing efforts to combat this global epidemic.
![]() References
References
[1] Drew B S, Dixon A F, Dixon J B. Obesity management: update on orlistat[J]. Vascular health and risk management, 2007, 3(6): 817-821.
[2] Ballinger A, Peikin S R. Orlistat: its current status as an anti-obesity drug[J]. European journal of pharmacology, 2002, 440(2-3): 109-117.
- Related articles
- Related Qustion
- Orlistat: Medical use, mechanism and side effects May 11, 2023
Orlistat is an internationally recognized new form of weight loss drug. Its commercial name is Sainike and first went on sale in New Zealand in 1998.
- Uses of Orlistat as a new form of Weight loss Drug Oct 21, 2019
Orlistat is an internationally recognized new form of weight loss drug. Its commercial name is Sainike and first went on sale in New Zealand in 1998.
Benorilate stands as a noteworthy advancement in pharmaceutical chemistry, combining the therapeutic benefits of salicylates with improved gastric tolerance.....
Jun 27,2024API3-Methacryloxypropyltrimethoxysilane (MPTMS), a chemical compound with the formula C10H20O5Si, holds a significant position in the realm of silane chemistry.....
Jun 27,2024APIOrlistat
96829-58-2You may like
- Orlistat
-

- $0.00 / 25KG
- 2024-07-27
- CAS:96829-58-2
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 20 TONS
- Orlistat
-

- $0.00 / 1Kg/Bag
- 2024-07-26
- CAS:96829-58-2
- Min. Order: 1Kg/Bag
- Purity: USP37 / 98.5%
- Supply Ability: 20 tons
- Orlistat
-
- $2.00 / 1kg
- 2024-07-26
- CAS:96829-58-2
- Min. Order: 0.10000000kg
- Purity: 99%
- Supply Ability: 200KG