Poly(tetrafluoroethylene): Properties and applications
Jan 12,2024
Description
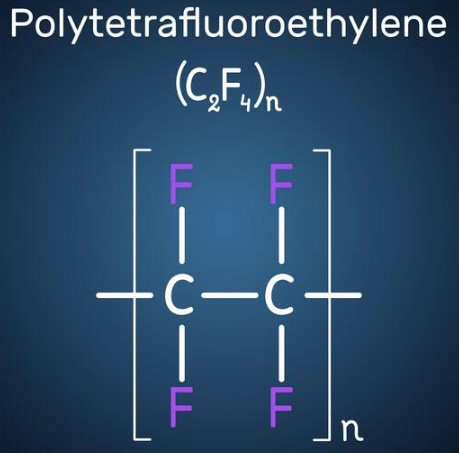
The basic monomer unit is a fluorinated ethylene molecule (—CF2 —CF2 —). It is well known under its common trade name, Teflon®. It was discovered in 1938 by Roy J. Plunkett, a DuPont scientist.
Preparation method
Industrially, polytetrafluoroethylene is obtained from several consecutive steps. First, chloroform reacts with hydrofluoric acid to yield chlorodifluoromethane. The chlorodifluoromethane is then pyrolyzed at 800–1000°C to yield the monomer, i.e., tetrafluoroethylene (CF2 =CF2, TFE), which is purified and polymerized in aqueous emulsion or suspension using organic peroxides, persulfates or hydrogen peroxide as catalysts. The simple polymerization reaction is as follows.
n (CF2 =CF2 ) → (—CF2 —CF2 —)n
Properties and applications
The macromolecule exhibits a structure with a high crystallinity that explains its unusually high melting point of 327°C. This characteristic ensures the highest useful temperature limit among plastics of 260°C. Polytetrafluoroethylene (PTFE) also exhibits good mechanical strength and an extremely low coefficient of friction that imparts excellent self-lubricating abilities to the material similar to those of graphite and molybdenum disilicide. PTFE exhibits the strongest anti-adhesive properties, and no compound is known to adhere durably to PTFE. However, PTFE remains the most difficult of the fluorocarbons to work with because of its high viscosity in the molten state; it cannot be cast, and extrusion requires particular procedures. Therefore, shapes and parts are usually made using powder metallurgy techniques such as sintering to produce them in usable forms. During sintering the PTFE powder or granules filling a mold of the desired shape are compressed under 14–70 MPa at a temperature well above 327°C and after cooling the PTFE exhibits the desired shape.
From a chemical point of view, PTFE is one of the most chemically inert materials known apart from glass, tantalum, platinum, and iridium for servicing (possessing a long service life) in various severely corrosive chemicals, even at high temperatures. Moreover, no organic or mineral solvent dissolves PTFE. The only chemicals known to attack PTFE readily are molten alkali metals (e.g., Li, Na, K) and nascent fluorine gas. Finally, PTFE has excellent insulating properties with a low loss tangent factor at high frequencies. Nevertheless, permeation is an issue depending on the specific exposure, but sometimes, it is just as good as many newer materials. Some problems associated with thermal cycling, which can cause fatigue due to repeated expansion and contraction over time when going through high temperatures, were reported.
Nevertheless, owing to their porosity, one particular mode of deterioration for fluorocarbons is the adsorption of a chemical, followed either by a reaction with another component inside the thermoplastic or by polymerization of the product within the plastic. When this phenomenon occurs, it leads to surface degradation, such as blistering. The material also has a definite heat limitation, and overheating should be avoided. The cold flow of the resins is well known, implying that the design and use of the fluorocarbon should be such that excessive compressive stresses are not imposed to create a cold flow condition. PTFE is now largely used in consumer goods as an anti-stick material. Industrially, its chemical inertness favors PTFE in applications involving harsh environments, such as piping, pump parts, and protective lining in the chemical process industry, while its self-lubricating properties allow its use in moving parts such as braking pads on machinery, rotors, and shafts packing, etc., where lubrication is an issue.
- Related articles
- Related Qustion
4,4'-Methylenedianiline(MDA) is primarily used to produce 4,4-'methylenedianiline diisocyanate and other polymeric isocyanates which are used to manufacture polyurethane foams.....
Dec 10,2024Organic ChemistrySilicon carbide (SiC), relative molar mass 40.097, is an important advanced ceramic with a high melting point (2830°C), a high thermal conductivity (135 Wm–1K–1), and extremely high Mohs hardness of 9.....
Jan 12,2024APIPoly(tetrafluoroethylene)
9002-84-0You may like
- The properties and toxicity assessment of homosalate
Mar 21, 2025
- Glycidol: both an epoxide and an alcohol
Mar 17, 2025
- Disposal and Utilization of Polyvinyl chloride
Mar 12, 2025
Poly(tetrafluoroethylene) manufacturers
- PTFE
-

- $0.00 / 25KG
- 2025-03-21
- CAS:9002-84-0
- Min. Order: 1KG
- Purity: 99.7%
- Supply Ability: 50000KG/month
- Poly(tetrafluoroethylene)
-

- $99.00 / 1kg
- 2025-03-21
- CAS:9002-84-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton
- Poly(tetrafluoroethylene)
-

- $9.90 / 1KG
- 2025-03-20
- CAS:9002-84-0
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 5tons






