Properties, pharmacological effects and clinical applications of clindamycin hydrochloride
Jul 25,2022
General description
Clindamycin, the 7(S)-chloro-7-deoxy derivative of lincomycin, has stood the test of time in the treatment of anaerobic infections. clindamycin also is a semisynthetic derivative of lincomycin and was introduced in the 1960s as an antibiotic. It is available as clindamycin hydrochloride for oral administration in capsules, as clindamycin phosphate for intramuscular or intravenous injection, and as clindamycin palmitate for oral suspensions [1-3]. When given orally, clindamycin is well absorbed and peak concentrations are found after about 45 min. It is metabolized into three major, biologically active derivatives and is mainly excreted into the bile, with about 20% excreted by the kidneys. The normal elimination half-life of about 2 to 4 h is not altered in patients with severe renal disease, but impaired liver function leads to a prolongation of elimination[4].
In addition, Clindamycin inhibits protein synthesis by acting on the 50S ribosomal subunits of bacteria. The colitis resulting from the use of clindamycin has been extensively studied and is now easily manageable. Although newer antibiotics active against anaerobes are available, clindamycin remains a reliable and well-tested antibiotic for use in anaerobic infections.
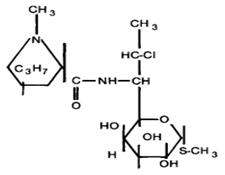
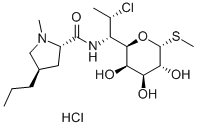
Fig. 1. Chemical structure of clindamycin hydrochloride.
Appliaction
Clindamycin has been extensively used in the therapy of obstetric and gynecologic infections for over 20 years. This antibiotic is well known for its activity against anaerobic bacteria, particularly β-lactamase-producing strains of the Bacteroides species [5]. Clinicians should also recognize its very good activity against aerobic gram-positive cocci, such as the group B streptococci, but be aware of its absence of activity against aerobic gram-negative rods, such as E.coli. In combination with an aminoglycoside, clindamycin has become the standard by which other antimicrobials have been judged in the treatment of pelvic infections [6].
Pharmacological activities
(1) Antimicrobial activity [7-9]
Clindamycin is about eight times more active than lincomycin against Staphylococcus aureus and Streptococcus pneumoniae. More than 900/0 of S. aureus strains are inhibited by 0.1 J-lg of clindamycin/ml. Clindamycin is four times more active than erythromycin against S. aureus and is active in vitro even against strains that are resistant to erythromycin, penicillin, and methicillin. At concentrations achievable in serum, clindamycin is active against Staphylococcus epidermidis, Streptococcus pyogenes, S. pneumoniae, and viridans Streptococcus. Group D streptococci like Streptococcus faecalis are usually resistant to this drug, whereas nonenterococcal group D streptococci like Streptococcus bovis are susceptible. Most strains of Corynebacterium diphtheriae are susceptible to clindamycin. (2) In vitro interaction with other antimicrobial agents
The antibacterial activity of clindamycin in the presence of other antibiotics has been studied, with somewhat conflicting results. Leng et al. reported synergy between clindamycin and gentamicin against E. coli. Other researchers found clindamycin and aminoglycosides to be synergistic in vitro against E. coli, Proteus mirabilis, and Pseudomonas aeruginosa. In another study, however, only half of 66 strains of Enterobacteriaceae and Pseudomonas were synergistically inhibited by clindamycin and gentamicin. Sabath and Toftegaard and Klastersky and Husson observed no such synergy. Zinner et al. noticed that clindamycin actually interfered with the early antibacterial activity of gentamicin and amikacin against E. coli and Klebsiella. The combination of clindamycin and an aminoglycoside was found to be synergistic against S. aureus, Streptococcus sanguis, and Streptococcus salivarius, but antagonistic with eight of nine strains of Streptococcus mutans. Clindamycin in combination with sulfamethoxazole and trimethoprim exerted only an additive effect against S. aureus, S. pyogenes, E. coli, Klebsiella, and Enterobacter. Similarly, no synergy was apparent when clindamycin was tested in combination with sulfamethoxazole or sulfisoxazole against H. influenzae.
(2) Treatment of hidradenitis suppurativa [10]
Thirty patients with recurrent suppurative hidradenitis entered a double blind trial to evaluate the effect of topical clindamycin against placebo in hidradenitis. Twenty seven patients concluded the 3 months' treatment. The overall effect of clindamycin treatment based on patients' assessments, number of abscesses, inflammatory nodules and pustules was significantly better than placebo at each monthly evaluation (P<0.01). When each parameter was evaluated separately, clindamycin was significantly superior to placebo except for inflammatory nodules and abscesses on the second and third months' evaluation. No side effects were recorded and the treatment was easy to administer. Topical clindamycin may be helpful prior to radical surgery or spontaneous remission.
(3) Skin and soft tissue infections [11]
With its activity against S aureus, streptococci and anaerobes, clindamycin has been found effective for various skin and soft tissue infections. Median minimum inhibitory concentration (MIC) for group A streptococci was 0.04 g/mL for clindamycin (range 0.02 to 0.1 g/mL), compared with 0.05 g/mL for penicillin G. For S aureus, median MIC was 0.1 g/mL compared with 0.4 g/mL for cloxacillin. Penicillin, cloxacillin or first-generation cephalosporins are usually preferred because of their lower cost, more reliable bactericidal activity and reduced propensity to cause C difficile-associated colitis. Strains of S aureus that are resistant to methicillin will also be resistant to clindamycin, while erythromycin-resistant strains have a tendency to acquire clindamycin resistance during therapy.
Mechanism of action
Clindamycin acts on the 50S ribosomal 'subunits of bacteria and inhibits protein synthesis. It primarily inhibits the initiation of peptide chain synthesis, whereas erythromycin prevents chain extension of growing peptides on the ribosomes, and chloramphenicol affects peptide bond formation [12]. Subinhibitory concentrations of lincomycin and clindamycin completely suppress the production of streptolysin S by bacteria. Clindamycin-mediated inhibition of bacterial protein synthesis has been noted to alter the bacterial surface in such a way that phagocytosis and intracellular killing of the bacteria is greatly facilitated. Although a similar effect on phagocytosis and killing is produced by other inhibitors of bacterial protein synthesis - e.g., erythromycin and chloramphenicol-it is distinctly absent with cell wall-active agents, such as penicillins and cephalosporins [13]. Clindamycin can potentiate opsonization and phagocytosis, even at subinhibitory concentrations.
Clindamycin during pregnancy
Clindamycin is generally considered safe for use by pregnant women. The pharmacokinetic properties of clindamycin remain unchanged during pregnancy. The drug readily crosses the placenta and is secreted in small amounts into human milk. A recent study with pregnant women compared a quinine-clindamycin regimen against an artesunate regimen and showed high cure rates and the safety of the regimen for both the mothers and the children [14]. In addition, three case reports that did not include a follow-up of the mothers or the children have described the use of clindamycin alone, quinine-clindamycin, or quinidine-clindamycin in pregnant women [15].
Safety
Although clindamycin is generally well tolerated, it can lead to the development of C. difficile-associated diarrhea, a common complication of antibiotic treatment. The risk of developing colitis with clindamycin treatment is similar to the risks with treatment with expanded-spectrum cephalosporins and broad-spectrum penicillins, and the rate of colitis is about 5% among hospitalized patients [16]. The risk increases by use of combinations of any of these drugs. The strongest risk factor for the disease is hospitalization, with outpatients having a fraction of the risk compared to that for inpatients. Other important factors are concomitant disease, advanced age, and the duration of treatment.
Difficile-associated colitis is rare in subjects taking clindamycin for 3 days or less. If clindamycin is discontinued immediately after the appearance of diarrhea, the disease is often self-limiting, and with appropriate treatment, severe morbidity or mortality is extremely rare [17-19]. Children are at far lower risk, and mortality was reported only during the era before identification of the cause of the disease and the introduction of an effective treatment. Few data are available on the prevalence of C. difficile in hospitals in regions where malaria is endemic. A study from Zambia suggests that it might be considerably less of a problem than it is in industrialized countries.
Medication for elderly patients
Clinical studies of clindamycin have not involved enough data in older adults to determine whether older people respond differently to the drug than younger people. There was no clinically significant difference in pharmacokinetics between young and old volunteers with normal liver and kidney function who received clindamycin orally or intravenously [20]. Diarrhea and antibiotic-associated colitis have been reported to be more common and severe in the elderly. Therefore, the elderly patients with diarrhea should be closely monitored.
References
[1] J. Zhang, High-efficiency algae remover and its application in algae removal in fish and shrimp breeding ponds, aquatic plant breeding ponds, decorative ponds and aquariums, Jiangsu Zhaozhiyuan Industrial Co., Ltd., Peop. Rep. China . 2021, p. 6pp.
[2] C. Zheng, Y. Chen, S. Liu, G. Shao, J. Jiang, Intelligent detection method of trichloromethyl carbonate residue in clindamycin hydrochloride [Machine Translation], Guangzhou Guobiao Inspection and Testing Co., Ltd., Peop. Rep. China . 2021, p. 20pp.
[3] Y. Shen, J. Yu, Z. Yang, Clindamycin hydrochloride injection and preparation method, Suzhou Tianma Pharma Group Tianji Bio-Pharmaceutical Co., Ltd., Peop. Rep. China . 2021, p. 5pp.
[4] C. Soonklang, C. Tassanarangsan, N. Soomherun, N. Kreua-Ongarjnukool, S.T. Niyomthai, Study kinetics models of clindamycin hydrochloride from poly(D,L-lactic-co-glycolic acid) particles, Int. J. Pharma Med. Biol. Sci. 10(2) (2021) 68-74.
[5] M.M. Abdellatif, Y.E. Elakkad, A.A. Elwakeel, R.M. Allam, M.R. Mousa, Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: In-vitro and in-vivo wound healing assessment, Saudi Pharm. J. 29(11) (2021) 1238-1249.
[6] S. Wang, B. Li, Preparation method of clindamycin hydrochloride palmitate dry suspension, Hainan Hishen Tongzhou Pharmaceutical Co., Ltd., Peop. Rep. China . 2021, p. 8pp.
[7] J. Wei, Y. Liu, S. Wang, Preparation method of high stability clindamycin hydrochloride palmitate granules, Hainan Hishen Tongzhou Pharmaceutical Co., Ltd., Peop. Rep. China . 2021, p. 7pp.
[8] Y. Feng, Y. Li, D. Zhou, B. Li, G. Chen, N. Li, Glycyrrhetinic acid reverses antibiotic-induced intestinal epithelial injury through RNA-binding protein human antigen R (HuR), Phytomedicine 94 (2022) 153836.
[9] P. O-chongpian, M. Na Takuathung, C. Chittasupho, W. Ruksiriwanich, T. Chaiwarit, P. Baipaywad, P. Jantrawut, Composite Nanocellulose Fibers-Based Hydrogels Loading Clindamycin HCl with Ca2+ and Citric Acid as Crosslinking Agents for Pharmaceutical Applications, Polymers (Basel, Switz.) 13(24) (2021) 4423.
[10] N. Pavlovic, I.A. Bogicevic, D. Zaklan, M. Djanic, S. Golocorbin-Kon, H. Al-Salami, M. Mikov, Influence of Bile Acids in Hydrogel Pharmaceutical Formulations on Dissolution Rate and Permeation of Clindamycin Hydrochloride, Gels 8(1) (2022) 35.
[11] H. Yan, J. Yu, X. Di, X. Wang, S. Liu, Y. Liu, Simultaneous determination of metronidazole and clindamycin hydrochloride in rabbit plasma by LC-MS/MS, Shenyang Yaoke Daxue Xuebao 38(12) (2021) 22-28.
[12] D.-d. Shen, Q. Wu, H.-y. Liang, Y. Cheng, C. Shen, Determination of the related substances in clindamycin hydrochloride and its preparations by HPLC, Zhongguo Kangshengsu Zazhi 46(5) (2021) 75-83.
[13] X.-b. Li, N. Wang, M. Yu, X.-j. Wu, R. Ma, Y.-t. Liu, Y. Zhou, H. Xie, X. Gao, W.-p. Wang, Bioequivalence study of clindamycin hydrochloride capsules in Chinese healthy subjects, Zhongguo Linchuang Yaolixue Zazhi 37(1) (2021) 10-13, 17.
[14] M.-l. Mao, P. Lin, L.-l. Xiong, Y. Feng, Q.-l. Shu, Changes in Diversity of Intestinal Butyrate-producing Bacteria During Treatment with Shenling Baizhusan and Lizhongtang in Animal Model of AAD, Zhongguo Shiyan Fangjixue Zazhi 27(22) (2021) 30-37.
[15] J. Zhang, J.-j. Zheng, Y. Wu, M. Wu, Efficacy and safety of clindamycin hydrochloride palmitate dispersion tablets for treatment of chronic periodontitis, Zhongguo Xinyao Zazhi 30(20) (2021) 47-50.
[16] Y. Guo, X. Yang, Y. Zhang, L. Qin, Separation and purification of clindamycin hydrochloride palmitate impurity using preparative liquid chromatography, Zhejiang Tiantai Pharmaceutical Co., Ltd., Peop. Rep. China . 2022, p. 11pp.
[17] J. He, R. Jia, G. Lv, F. Xiao, Pharmaceutical suppository composition-containing tinidazole, its preparation method and application, Jiangsu Yuanheng Pharmaceutical Co., Ltd., Peop. Rep. China . 2022, p. 13pp.
[18] X.-y. Hou, J.-m. Gao, D.-r. Wang, J.-s. Guo, L.-x. Zhang, T. Zhu, Residue levels and distribution characteristics of antibiotics in the soil and water environment of Shenzha Town, Tibet, Zhongguo Huanjing Kexue 41(12) (2021) 5849-5856.
[19] J.-h. Liu, X.-m. Tang, L.-y. Song, S. Wang, Improvement of bacterial endotoxins testing method for clindamycin hydrochloride injection, Harbin Shangye Daxue Xuebao, Ziran Kexueban 35(4) (2019) 388-391.
[20] R.E. Hughes, R.J.R. Elliott, X. Li, A.F. Munro, A. Makda, R.N. Carter, N.M. Morton, K. Fujihara, N.J. Clemons, R. Fitzgerald, J.R. O'Neill, T. Hupp, N.O. Carragher, Multiparametric High-Content Cell Painting Identifies Copper Ionophores as Selective Modulators of Esophageal Cancer Phenotypes, ACS Chem. Biol. 17(7) (2022) 1876-1889.
- Related articles
- Related Qustion
Vericiguat is a stimulator of soluble guanylate cyclase (sGC),it is used to reduce the risk of cardiovascular death and hospitalization for heart failure following a prior hospitalization for heart failure....
Jul 22,2022APICommon preparations of polymyxin B sulfate primarily include injections. It is used to resist the infection caused by gram-negative bacilli, mainly Pseudomonas aeruginosa.....
Jul 25,2022APIClindamycin hydrochloride
21462-39-5You may like
Clindamycin hydrochloride manufacturers
- Clindamycin hydrochloride
-

- $0.00 / 25kg
- 2024-07-23
- CAS:21462-39-5
- Min. Order: 25kg
- Purity: >800ug/mg
- Supply Ability: 10tons
- Clindamycin hydrochloride
-

- $70.00 / 100kg
- 2024-07-01
- CAS:21462-39-5
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1t
- Clindamycin hydrochloride
-

- $0.00 / 1KG
- 2024-06-14
- CAS:21462-39-5
- Min. Order: 1KG
- Purity: USP, GMP
- Supply Ability: 5,000KG