Cetuximab: Bioactivity, Drug resistance, Safety and toxicological studies
Dec 26,2022
General description
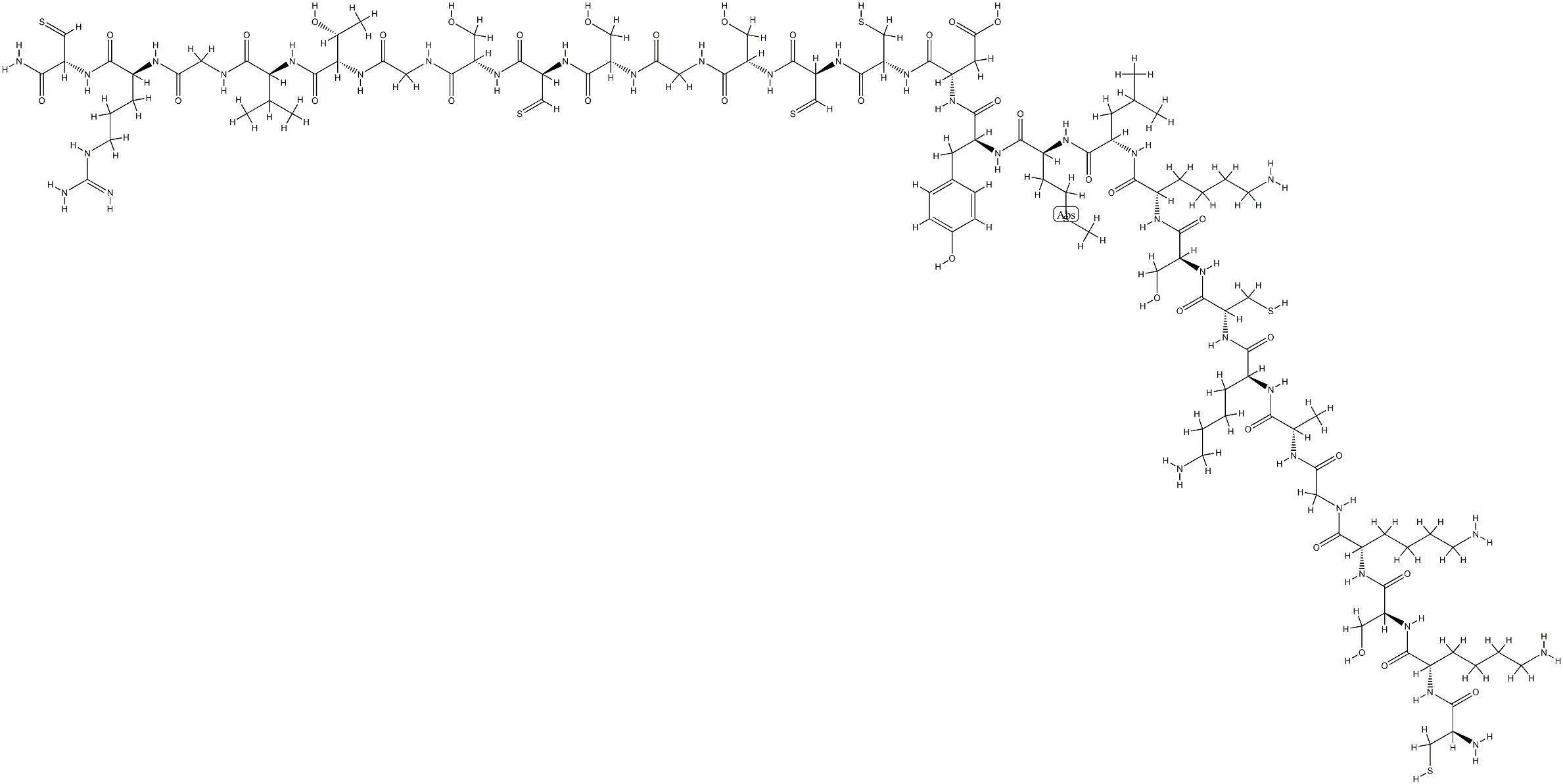
The Cetuximab, it’s molecular formula is C107H179N35O36S7 and molecular weight is 2756.23406 and the following structure.

Figure 1 the molecular formula of cetuximab
Indication
Cetuximab is a human IgG1 monoclonal antibody that inhibits epidermal growth factor receptor (EGFR), with a Kd of 0.201 nM for EGFR by SPR and has potent antitumor activity.[1]
Pharmacodynamics
In Vitro:Cetuximab is a monoclonal antibody that inhibits epidermal growth factor receptor (EGFR), with a Kd of 0.201 nM for soluble EGFR by SPR. Cetuximab also exhibits a Kd of 0.147 nM for EGFR in fixed A431 cells by ELISA.Cetuximab (C225; 30 nM) time-dependently inhibits the proliferation of SCC-1, SCC-11B, SCC-38, and SCC-13Y cells after treatment for 8 d. Cetuximab (30 nM) causes G0/G1 arrest, induces apoptosis, and reduces Rb, p27KIP1, Bcl-2, and Bax expression in SCC-13Y cells. Cetuximab (30 nM) also enhances radiosensitivity and increases radiation-induced apoptosis in SCC-13Y cells.[2] In Vivo:Cetuximab (1 mg/injection) has effect on the tumour volume but the effect is more pronounced on UT-SCC-14 xenografts. In UT-SCC-14 xenografts, Cetuximab treatment significantly reduces the expression of EGFR, pEGFR and Ki67. The MCT1 and GLUT1 expression is significantly decreased in the Cetuximab-treated groups of both cell lines but differences are more pronounced in UT-SCC-14 xenograft.[3]
Clinical application
Cetuximab can be used to treat colorectal cancers, which lead to dynamic increase of M2 macrophages with disease progression during the treatment.[4] Combinate chemotherapies (irinotecan, fluoropyrimidines and oxaliplatin) with Cetuximab have improved clinical responses and survival of mCRC. [5] What’s more, cetuximab, as an anti-epidermal growth factor receptor (EGFR) drug, as second- or third-line treatment in non-small cell lung cancer (NSCLC) patients.Cetuximab plus avelumab treatment induced additive cancer cell growth inhibition as compared to single agent treatment. This effect was partially blocked by treatment with an anti-CD16 mAb, suggesting a direct involvement of NK cell activation. Furthermore, cetuximab plus avelumab treatment induced 10-, 20-, and 20-fold increase, resp., in the gene expression of CCL5 and CXCL10, two STING downstream effector cytokines, and of interferon β, as compared to untreated control samples. [6]
Drug resistance
Cetuximab is currently the only EGFR-targeting agent approved by the FDA for treatment of HNC; however, intrinsic and acquired resistance to cetuximab is a major problem in the clinic. The previous research result that AXL leads to cetuximab resistance via activation of HER3.This test demonstrated that addition of the HER3 ligand NRG1 to cetuximab-sensitive HNC cells leads to cetuximab resistance. Further, AXL-overexpressing cells regulate NRG1 at the level of transcription, thereby promoting cetuximab resistance. Immunoblot anal. revealed that NRG1 expression was relatively high in cetuximab-resistant HNC PDXs compared to cetuximab-sensitive HNC PDXs. Finally, genetic inhibition of NRG1 resensitized AXL-overexpressing cells to cetuximab. The results of this study indicate that AXL may signal through HER3 via NRG1 to promote cetuximab resistance .[7] The findings identified MIR100HG as a potent Epithelial-to-mesenchymal transition (EMT) inducer in CRC that may contribute to cetuximab resistance and metastasis by activation of a MIR100HG/hnRNPA2B1/TCF7L2 feedback loop.[8] In addition, Cetuximab resistance in colorectal cancer (CRC) is responsible to poor prognosis to some extent. M2 macrophage polarization is closely correlated with drug resistance to cancers in investigation about whether the mechanism of HCG18 on cetuximab resistance to CRC involving in M2 macrophage polarization. The results revealed that HCG18 promoted M2 macrophage polarization to facilitate cetuximab resistance to CRC cells through modulating miR-365a-3p/FOXO1/CSF-1 axis.[9]
Safety
Cetuximab is a kind of drug with excellent tolerability in the eyes, there is a study showed that repeated intravitreal application of cetuximab did not result in any detected intraocular toxic or destructive effect in young and adult rabbits.[10]
Toxicity
The majority of skin toxicities were mild to moderate, symptomatic treatments were effective in controlling them. The skin discomfort caused by cetuximab can affect the quality of life temporarily; with long term treatment, severity of skin toxicity may decrease.[11]
References
1. Goldstein NI, et al. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995 Nov;1(11):1311-8.
2.Huang SM, et al. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999 Apr 15;59(8):1935-40.
3.Gustafsson H, et al. EPR Oximetry of Cetuximab-Treated Head-and-Neck Tumours in a Mouse Model. Cell Biochem Biophys. 2017 Jul 29.
4.Kim, Hyung-Don; Kim, Sun Young; Kim, Jihun; Kim, Jeong Eun; Hong, Yong Sang; Han, Buhm; Tak, Eunyoung; Ryu, Yeon-Mi; Kim, Sang-Yeob; Kim, Tae Won.Dynamic increase of M2 macrophages is associated with disease progression of colorectal cancers following cetuximab-based treatment[J].cientific Reports, 2022, 12(1):1678.
5.Ottaiano A, Scala S, Normanno N, et al. Cetuximab, irinotecan and fluorouracile in fiRst-line treatment of immunologicallyselected advanced colorectal cancer patients: the CIFRA study protocol. BMC Cancer. 2019;19(1):899.
6.Della Corte, Carminia Maria; Fasano, Morena; Ciaramella, Vincenza; Cimmino, Flora, et al. Anti-tumor activity of cetuximab plus avelumab in non-small cell lung cancer patients involves innate immunity activation: findings from the CAVE-Lung trial[J].Journal of Experimental & Clinical Cancer Research (2022), 41(1), 109 .
7. Iida M, Mcdaniel N K, Kostecki K L, et al. AXL regulates neuregulin1 expression leading to cetuximab resistance in head and neck cancer[J]. BMC Cancer, 2022, 22(1):1-13.
8.Liu H, Li D, Sun L, et al. Interaction of lncRNA MIR100HG with hnRNPA2B1 facilitates m6A-dependent stabilization of TCF7L2 mRNA and colorectal cancer progression[J]. Molecular Cancer, 2022, 21(1):1-18.
9.Gao, Cao; Hu, Wenwei; Zhao, Jiemin; Ni, Xuefeng; Xu, Yanjie.LncRNA HCG18 promotes M2 macrophage polarization to accelerate cetuximab resistance in colorectal cancer through regulating miR-365a-3p/FOXO1/CSF-1 axis[J].Pathology, Research and Practice.2022,240.
10.Bikbov, Mukharram M.; Kazakbaeva, Gyulli M.; Panda-Jonas, Songhomitra; Khakimov, Dinar A, et al. Safety and tolerability of intravitreal cetuximab in young and adult rabbits.[J]Scientific Reports.2022,12(1).
11.Bekouaci S, Smaili F. P-380Skin toxicity of cetuximab in patients with metastatic colorectal carcinoma (mCRC)[J]. Annals of Oncology, 2019, 30(Supplement_4).
- Related articles
- Related Qustion
- The Uses and indications Cetuximab Apr 12, 2024
Cetuximab is a chimeric human-murine monoclonal antibody against EGFR.
- Cetuximab: A Beacon in Targeted Cancer Therapy and Precision Medicine Apr 12, 2024
Cetuximab stands at the forefront of targeted cancer therapies, embodying the advances in precision medicine that have revolutionized oncological treatment.
Prednisolone is a medicine used to treat a wide range of health problems including allergies, blood disorders, skin diseases, inflammation, infections and certain cancers and to prevent organ rejection after a transplant. It helps by reduci....
Dec 23,2022APIThe 3-isopropylphenol, is also known as 3-isopropylbenzenesulfonyl chloride. It belongs to the Phenolic compound and as a ingredient of disinfectants.....
Dec 26,2022APICetuximab
205923-56-4You may like
- CETUXIMAB USP/EP/BP
-

- $1.10 / 1g
- 2021-07-03
- CAS:205923-56-4
- Min. Order: 1g
- Purity: 99.9%
- Supply Ability: 100 Tons Min
- Cetuximab
-

- $0.00 / 1Kg
- 2020-05-03
- CAS:205923-56-4
- Min. Order: 1KG
- Purity: 99.0%
- Supply Ability: 800 ton
- CETUXIMAB
-

- $1.00 / 1KG
- 2020-03-06
- CAS:205923-56-4
- Min. Order: 1KG
- Purity: 99%
- Supply Ability: 200kgs