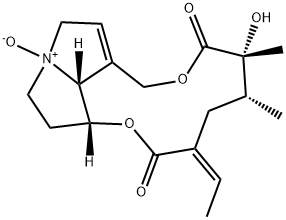
Senecionine N-oxide – a Tertiary Amine Oxide
Dec 16,2019
Senecionine N-oxide is a tertiary amine oxide. It derives from a senecionine.
Root cultures of Senecio vulgaris synthesize pyrrolizidine alkaloids which are accumulated in the form of their N-oxides. The cultures incorporate biosynthetic precursors, such as arginine, ornithine, isoleucine, putrescine and spermidine. with high efficiency into the alkaloids. Senecionine N-oxide is found to be the primary product of biosynthesis. With putrescine and spermidine incorporation rates of 20-30 % are obtained. The N-oxide synthesized does not appear to undergo significant turnover. Tertiary pyrrolizidine alkaloids, if found at all, occur in small amounts in old tissues only. They are derived from the corresponding oxides, and are easily formed spontaneously during alkaloid extraction. The suitability of N-oxides in alkaloid storage is discussed [1].

Plant defenses against herbivores and insects range from morphological modifications to biosynthesis of toxic compounds. Pyrrolizidine alkaloids (PA) are one such group of plant defense compounds that occur in a wide range of plants, but more so in Asteraceae and Boraginaceae. The high toxicity of Senecio species is due to high concentrations of pyrrolizidine alkaloids in the plant tissues. The major product is senecionine N-oxide and the main site of biosynthesis is the roots of the plants.
A gene duplication of a metabolic enzyme from primary metabolism followed by diversification and further modifications is believed to be the basis for the evolution of these compounds. The underlying biosynthesis of pyrrolizidine alkaloids have been extensively studied in insect species which are known to have recruited these compounds for their own defense. They have also had to develop complex mechanisms to protect themselves from the toxicity of PAs . One of the most common methods of detoxification is by N-oxidation of the pyrrolizidine alkaloids. A gene duplication of pyrrolizidine alkaloid N-oxygenases in adapted Lepidoptera moths is traced back to an ancestral flavin monooxygenase of unknown function. Estigmene acrea is able to sequesters all known pyrrolizidine alkaloids from all major plant families known produce PAs. Insect adaptation strategies include (i) pyrrolizidine alkaloid-specific taste receptors; (ii) detoxification of pyrrolizidine alkaloids by N-oxidation, catalyzed by a specific flavin-dependent monooxygenase; (iii) transfer and maintenance of all types of pyrrolizidine N-oxides through all developmental stages[2].

References
[1] THOMAS HARTMANN and GERD TOPPEL, SENECIONINE N-OXIDE, THE PRIMARY PRODUCT OF PYRROLIZIDINE ALKALOID BIOSYNTHESIS IN ROOT CULTURES OF SENECIO VULGARIS, Phymhentiay. Vol. 26, No. 6, pi. 1639-1643, 1987.
[2] https://biocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY-6852
- Related articles
- Related Qustion
Davy and Brande independently prepared lithium metal in 1818 by the electrolysis of lithium oxide. However, larger quantities of the metal were prepared by Bunsen and Mattiessen in 1855 by electrolysis of the chloride. Guntz first proposed....
Dec 16,2019Inorganic chemistryGlucoraphanin is a glucosinolate found in broccoli, cauliflower, and mustard. Glucoraphanin is converted to sulforaphane by the enzyme myrosinase. In plants, sulforaphane deters insect predators and acts as a selective antibiotic.....
Dec 16,2019Plant extractssenecionine N-oxide
13268-67-2You may like
- What is shellac used for?
Jul 1, 2024
- What are the uses of Troxerutin?
Jun 20, 2024
- The side effects and Precautions of Lycopene
Jun 13, 2024