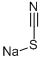Sodium Thiocyanate: A Comprehensive Guide for Chemical Industry Professionals
Apr 30,2024
Introduction
Sodium thiocyanate (NaSCN) is a pivotal chemical compound extensively utilized across various industrial sectors, including pharmaceuticals, textiles, agriculture, and electroplating. This article aims to provide a comprehensive analysis of sodium thiocyanate, covering its synthesis, chemical structure, main components, broad applications, optimal storage methods, and essential safety precautions. As a versatile reagent, sodium thiocyanate plays a critical role in numerous chemical reactions and manufacturing processes. Understanding the complex properties and effective handling of NaSCN is crucial for professionals in the chemical industry to ensure its optimal use while maintaining safety standards. This in-depth exploration will highlight the significance of sodium thiocyanate in advancing industrial capabilities and enhancing product development.

Figure 1 Characteristics of Sodium thiocyanate
Synthesis of Sodium Thiocyanate
Sodium thiocyanate is typically synthesized through the reaction of cyanide salts, such as sodium cyanide (NaCN), with elemental sulfur or thiocyanate salts. This process involves heating sodium cyanide in the presence of sulfur at high temperatures, a method that facilitates the formation of thiocyanate anions.
This reaction not only yields sodium thiocyanate but also poses significant risks due to the toxic nature of cyanides. Therefore, stringent safety measures are critical during production to mitigate potential hazards[1].
Main Components
Sodium thiocyanate is characterized by its simple composition, primarily consisting of a sodium ion (Na+) and a thiocyanate ion (SCN-). This ionic compound is known for its high solubility in water and its hygroscopic properties, making it a versatile reactant in various chemical reactions. The purity of commercially available sodium thiocyanate typically exceeds 98%, making it a reliable compound in high-stakes industrial applications.
Applications of Sodium Thiocyanate
The applications of sodium thiocyanate are diverse and impactful within the chemical industry. Some of the primary uses include:
Chemical Synthesis
It serves as a precursor and a reagent in the synthesis of pharmaceuticals, agricultural chemicals, and other organic compounds.
Textile and Fiber Industries
Sodium thiocyanate is used as a spinning solvent in the manufacture of polyacrylonitrile fibers and as an anti-caking agent in the textile industry.
Metal Processing
It functions as a corrosion inhibitor and a metal extraction agent in metallurgical processes.
Photographic Applications
Utilized in photographic solutions to increase the solubility of developing agents.
Each of these applications leverages the unique properties of sodium thiocyanate, particularly its reactivity and solubility[2].
Storage Methods
Storing sodium thiocyanate requires careful consideration to maintain its stability and ensure safety. The compound should be kept in a cool, dry place away from direct sunlight. It is typically stored in tightly sealed containers made of materials that do not react with thiocyanates, such as high-density polyethylene (HDPE). Proper labeling and adherence to local regulations regarding hazardous materials are essential components of effective storage practices.
Safety Precautions
Handling sodium thiocyanate with appropriate safety measures is crucial due to its potential health hazards. It is moderately toxic by ingestion, and contact with skin or eyes can cause irritation. Therefore, protective clothing, gloves, and eye protection are mandatory when working with this compound. Additionally, ensuring adequate ventilation in the working area helps to mitigate any risks associated with its fumes or dust.
In case of a spill, emergency procedures should be implemented promptly. This involves evacuating the area, avoiding dust formation, and neutralizing the spilled compound using suitable agents like sodium bicarbonate.
Conclusion
Sodium thiocyanate is a significant chemical in the industrial landscape, marked by its versatile applications and critical role in synthesis processes. By understanding its properties, synthesis methods, and the precautions necessary for its use, professionals in the chemical industry can safely and effectively incorporate sodium thiocyanate into their operations. The ongoing study and responsible handling of this compound will continue to be of paramount importance in advancing industrial and scientific endeavors.
References
[1]Van Rooyen P H, Boeyens J C A. Sodium thiocyanate[J]. Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, 1975, 31(12): 2933-2934.
[2]Almeida J D, Bradburne A F, Wreghitt T G. The effect of sodium thiocyanate on virus structure[J]. Journal of Medical Virology, 1979, 4(4): 269-277.
- Related articles
- Related Qustion
- Understanding the Diverse Applications and Potential Hazards of Sodium Thiocyanate Jan 3, 2024
Sodium thiocyanate is a white crystalline powder and highly soluble in water, ethanol, and acetone. It has various industrial applications but poses health risks and environmental hazards.
- Sodium thiocyanate: pharmacological activity and toxicity Jun 28, 2023
Sodium thiocyanate has various pharmacological activities. However, it's important to note that sodium thiocyanate can be toxic, causing gastrointestinal, neurologic, and cardiovascular effects.
- Sodium thiocyanate-Application Dec 12, 2019
Sodium thiocyanate is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion.
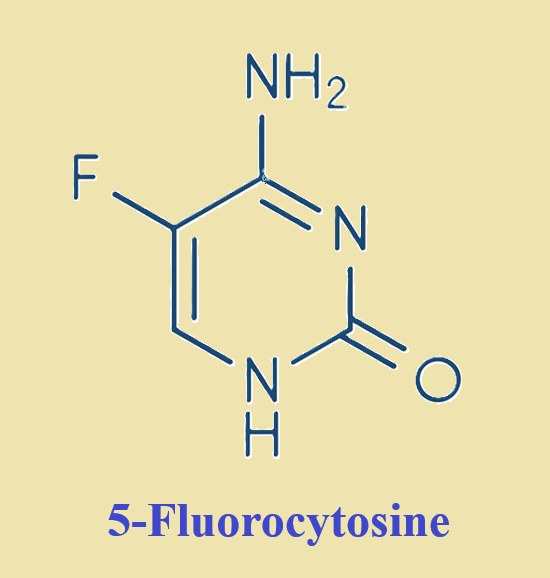
Yes. The therapeutic fluoropyrimidines 5-fluorouracil (5-FU) and 5-fluorocytosine (5-FC) have long been used to treat human cancer and severe invasive fungal infections, respectively.....
Nov 1,2024Biochemical EngineeringSelenium dioxide, a white solid, aids ZnSe nanocrystal synthesis, removes SO2 from flue gas, and highlights versatility despite toxicity concerns.....
Apr 30,2024APISodium thiocyanate
540-72-7You may like
- What is the nitration process of Methyl benzoate?
Nov 2, 2024
- What are the Benefits of Tributyrin?
Nov 2, 2024
- What is Anisole used as?
Nov 2, 2024
Sodium thiocyanate manufacturers
- Sodium thiocyanate
-
- $1000.00/ kg
- 2024-11-03
- CAS:540-72-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 5000
- Sodium thiocyanate
-

- $0.00 / 25KG
- 2024-11-02
- CAS:540-72-7
- Min. Order: 25KG
- Purity: 98%min
- Supply Ability: 30tons/month
- Sodium Thiocyanate
-

- $99.00 / 1kg
- 2024-11-01
- CAS:540-72-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20ton