Sulforaphene – Pharmacological action and its Application in Cancer Treatment
Dec 16,2019
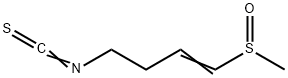
Sulforaphene, or raphanin, is the main sulfur component found in radish seeds of Raphanus sativus and is also found in broccoli and red cabbage [1]. It was first described by G. Ivanovics and S. Horvath in 1947 [2]. Sulforaphene inhibits activity of viruses, some fungi and various bacteria including Staphylococcus, Streptococcus, Pneumococcus and Escherichia coli. The effect is stronger against Gram-positive than Gram-negative bacteria and against DNA than RNA viruses; it is suppressed by serum and by sulfur compounds such as hydrogen sulfide, mercaptoacetic acid, cystine and glutathione. The antibacterial, antifungal and antiviral effects from consuming radishes were recognized in traditional Chinese medicine. However, in the abstract to his 1947 paper, Ivanovics noted that because sulforaphene is highly toxic, it did not "hold the promise of a useful therapeutic agent".

Sulforaphene may be a potential anti-triple negative breast cancer natural compound and its antiproliferation effects may be mediated by tumor suppressor Egr1; it has chemotherapeutic potential, it promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 down-regulation; it enhances radiosensitivity of hepatocellular carcinoma through suppression of the NF-κB pathway. Sulforaphene has anti-proliferative and antibacterial properties. Sulforaphene also has herbicidal activity, the ED50 of it against velvetleaf seedlings was approximately 2×10-4 M.
Lung cancer is one of the leading causes of cancer death worldwide. Isothiocyanates from cruciferous vegetables been shown to possess anticarcinogenic activities in lung malignances. Ming Yang etc. previously found sulforaphene (4-methylsufinyl-3-butenyl isothiocyanate, SFE), one new kind of isothiocyanates, existing in a relative high abundance in radish seeds. An efficient methodology based on macroporous resin and preparative high-performance liquid chromatography was developed to isolate SFE in reasonably large quantities, high purity and low cost. However, it is still largely unclear whether SFE could function as an antineoplastic compound, especially in lung cancer. Ming Yang etc. systematically investigated the anti-cancer effects of SFE in vitro as well as its possible underling molecular mechanisms in lung cancer. The acute toxicity tests and pharmacokinetics tests for SFE were performed to evaluate its drugability in mice. Also, they evaluated the in vivo anti-cancer effects of SFE using nude Balb/C mice with lung cancer xenograft. SFE can induce apoptosis of multiple lung cancer celllines and, thus, inhibited cancer cell proliferation. Lung cancer cells treated with SFE exhibit significant inhibition of the PI3K-AKT signaling pathway, including depressed PTEN expression and inhibition of AKT phosphoralation. At well-tolerated doses, administration of SFE to mice bearing lung cancer xenografts leads to significant inhibitions of tumor growth. In summary, our work identifies SFE as a novel natural broad-spectrum small molecule inhibitor for lung cancer [4].
Isothiocyanates derived from the Brassicaceae plants possess chemopreventive and anticancer activities. One of them is sulforaphene, which is abundant in Rhapanus sativus seeds. The underlying mechanism of its anticancer activity is still underexplored. Sulforaphene properties make it an interesting candidate for cancer prevention and therapy. Thus, it is crucial to characterize the mechanism of its activity. Anna Pawlik etc. investigated the mechanism of antiproliferative activity of Sulforaphene in breast cancer cells differing in growth factor receptor status and lacking functional p53. Viability of SKBR-3 and MDA-MB-231 breast cancer cells treated with sulforaphene was determined by SRB and clonogenic assays. Cell cycle, cell death and oxidative stress were analyzed by flow cytometry or microscopy. The levels of apoptosis and autophagy markers were assessed by immunoblotting. Sulforaphene efficiently decreased the viability of breast cancer cells, while normal cells (MCF10A) were less sensitive to the analyzed isothiocyanate. Sulforaphene induced G2/M cell cycle arrest, as well as disturbed cytoskeletal organization and reduced clonogenic potential of the cancer cells. Sulforaphene induced apoptosis in a concentration-dependent manner which was associated with the oxidative stress, mitochondria dysfunction, increased Bax:Bcl2 ratio and ADRP levels. Sulforaphene also potentiated autophagy which played a cytoprotective role. Sulforaphene exhibits cytotoxic activity against breast cancer cells even at relatively low concentrations (5-10 μM). This is associated with induction of the cell cycle arrest and apoptosis. Sulforaphene might be considered as a potent anticancer agent [5].
References
[1] Sinha, Nirmal K.; Hui, Y. H.; Muhammad Siddiq; Jasim Ahmed (2010). Handbook of Vegetables and Vegetable Processing. John Wiley and Sons. p. 156. ISBN 978-0-8138-1541-1.
[2] Ivãnovics, G. & S. Horvãth (1947). "Raphanin, an Antibacterial Principle of the Radish (Raphanus sativus)". Nature. 160 (4061): 297–298. doi:10.1038/160297a0. PMID 20261763.
[3] https://en.wikipedia.org/wiki/Raphanin
[4] Ming Yang, Haiyong Wang, Mo Zhou, Weilin Liu, Pengqun Kuang, Hao Liang, Qipeng Yuan, The natural compound sulforaphene, as a novel anticancer reagent, targeting PI3K-AKT signaling pathway in lung cancer, Oncotarget, Vol. 7, No. 47, 76656- 76666.
[5] Anna Pawlik, Marta Wała, Aleksandra Hać, Agnieszka Felczykowska, Anna Herman Antosiewicz, Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells, Phytomedicine. 2017 Jun 15;29:1-10. doi: 10.1016/j.phymed.2017.03.007.
- Related articles
- Related Qustion
- Sulforaphene: pharmacokinetics, activities and safety Dec 6, 2023
Sulforaphene, found in cruciferous vegetables, has complex metabolism, rapid absorption, and potential health benefits against cancer and cardiovascular disease.
Chelidonine, also named ranunculine, is a benzophenanthridine alkaloid isolated from the whole plant of Chelidonium majus. L. It has been used as a painkiller in clinical treatment based on its morphine-like analgesic effect.....
Dec 16,2019Antipyretic analgesicsDavy and Brande independently prepared lithium metal in 1818 by the electrolysis of lithium oxide. However, larger quantities of the metal were prepared by Bunsen and Mattiessen in 1855 by electrolysis of the chloride. Guntz first proposed....
Dec 16,2019Inorganic chemistrySulforaphene
592-95-0You may like
- What are the benefits of Rutin
Aug 7, 2024
- The introduction of Ingenol mebutate
Jul 30, 2024
- Hydroxyethyl starch: Uses, Elimination, and Side effects
Jul 29, 2024
- Sulforaphene
-

- $0.00 / 1kg
- 2024-04-25
- CAS:592-95-0
- Min. Order: 1kg
- Purity: >98%
- Supply Ability: 1000 kg
- Sulforaphene
-
- $0.00 / 20mg
- 2023-02-24
- CAS:592-95-0
- Min. Order: 20mg
- Purity: ≥98%(HPLC)
- Supply Ability: 10 g
- Sulforaphene
-

- $3.00 / 1KG
- 2020-01-13
- CAS:592-95-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 200KG