Synthesis and Application of 2-Chloro-3-Iodine-4-Pyridinamine
Nov 24,2022
General description
2-Chloro-3-Iodine-4-Pyridinamine is a solid with a melting point of 116-117℃. The predicted boiling point and density are 377.0±42.0 °C and 2.139±0.06 g/cm3, respectively. It needs to be kept at room temperature in a dry place. 2-Chloro-3-Iodine-4-Pyridinamine can be used as organic intermediates and pharmaceutical intermediates, mainly used in laboratory research and development process and chemical pharmaceutical analysis process.
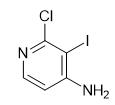
Fig. 1 The structure of 2-Chloro-3-Iodine-4-Pyridinamine.
Synthetic routes
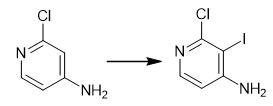
Fig. 2 The synthetic method of 2-Chloro-3-Iodine-4-Pyridinamine.
Add iodine monochloride (27.8 g, 171 mmol) in AcOH (10 ml) dropwise to a solution of 2-chloropyridin-4-amine (20.0 g, 156 mmol), NaOAc.3H2O (31.7 g, 233 mmol) in AcOH (170 ml). Heat at 70°C with stirring under N2 for 16 h. Extract reaction mixture with EtOAc (100 ml). Wash with Na2CO3 solution, 10% Na2S2O3 and brine. Combine the organic layers and dry over Na2SO4. Remove the solvent under reduced pressure. Purify the crude obtained by flash column chromatography (eluents: 40% EtOAc/hexane). 1H NMR (400 MHz, DMSO-d6) δ 7.74 (d, J= 5.3 Hz, 1H), 6.53 (d, J= 5.3 Hz, 3H) [1].
Application
Synthesis of new pyrrolo[3,2-c]pyridine Mannich bases
Jose’s et al describe the synthesis and biological evaluation of a new series of pyrrolo[3,2-c]pyridine Mannich bases (7a-v). The Mannich bases were obtained in good yields by one-pot three component condensation of pyrrolo[3,2-c]pyridine scaffold (6a-c) with secondary amines and excess of formaldehyde solution in AcOH. The chemical structures of the compounds were characterized by 1H NMR, 13C NMR, LC/MS and elemental analysis. Single crystal X-ray diffraction has been recorded for compound 7k ([C23H29ClN4]+2, H2O). The in vitro antimicrobial activities of the compounds were evaluated against various bacterial and fungal strains using Agar diffusion method and Broth micro dilution method. Compounds 7e, 7f, 7r, 7t, and 7u were showed good Gram-positive antibacterial activity against S. aureus, B. flexus, C. sporogenes and S. mutans. Furthermore, in vitro antimycobacterial activity was evaluated against Mycobacterium tuberculosis H37Rv (ATCC 27294) using MABA. Compounds 7r, 7t, and 7u were showed good antitubercular activity against Mtb (MIC ≥6.25 μg/mL). Among the tested compounds, 1-((4-chloro-2-(cyclohexylmethyl)-1H-pyrrolo[3,2-c]pyridin-3-yl)methyl)piperidine-3-carboxamide (7t) was showed excellent antimycobacterial activity against Mtb (MIC <0.78 μg/mL) and low cytotoxicity against the HEK-293T cell line (SI >>25). Molecular docking of the active compounds against glutamate racemase (MurI) and Mtb glutamine synthetase were explained the structure-activity observed in vitro [2].
Synthesis of 4-substituted 2-(1H-pyrrolo[3,2-c]pyridin-2-yl)propan-2-ols
A library of 16 4-substituted 2-(1H-pyrrolo[3,2-c]pyridin-2-yl)propan-2-ols 17–32 has been synthesized for use in biological testing against Trypanosoma cruzi, the protozoan parasite that causes Chagas disease. The 4-substituted 2-(1H-pyrrolo[3,2-c]pyridin-2-yl)propan-2-ols 17–32 were subjected to biological testing to evaluate their efficacy against intracellular Trypanosoma cruzi (Y strain) amastigotes infecting U2OS human cells, with benznidazole as a reference compound. The assay was performed in duplicate (two independent experiments) and submitted to High Content Analysis (HCA) for determination of trypanocidal activity. Three of the tested compounds presented relatively high trypanocidal activity (19, 22 and 29), however severe host cell toxicity was observed concomitantly. Chemical optimization of the highly active compounds and the synthesis of more compounds for biological testing against Trypanosoma cruzi will be required to improve selectivity and so that a structure-activity relationship can be generated to provide a more insightful analysis of both chemical and biological aspects [3].
Synthesis of new pyrrolo[3,2-c]pyridine derivatives
New antibacterial agents, pyrrolo[3,2-c]pyridine derivatives have been designed and synthesized. The structural considerations of the designed molecules were further supported by the docking study with GlcN-6-P synthase. The chemical structures of the new compounds were characterized by NMR, mass spectral analysis and elemental analysis. Single crystals of two compounds, C13H15N2Cl [6a] and C21H24N3OCl, CH4O [7c] were obtained allowing for structural analysis. [C13H15N2Cl] monoclinic, P21/c, a = 9.9763(6) ?, b = 9.6777(6) ?, c = 13.3002(9) ?, β = 106.459(7)°, V = 1231.47(14) ?3, Z = 4, T = 173(2) K, μ(Cu Kα) = 2.522 mm?1, Dcalc = 1.266 g/mm3, 7124 reflections, 2404 unique (Rint = 0.0381), R1 = 0.0420 (I > 2σ(I)) and wR2 = 0.1254 (all data). [C21H24N3OCl, CH4O] triclinic, P?1, a = 10.1478(7) ?, b = 12.0945(8) ?, c = 18.3244(10) ?, α = 104.369(5)°, β = 90.766(5)°, γ = 99.235(6)°, V = 2147.1(2) ?3, Z = 4, T = 173(2) K, μ(Cu Kα) = 1.744 mm?1, Dcalc = 1.243 g/mm3, 14238 reflections, 8297 unique (Rint = 0.0330), R1 = 0.0578 (I > 2σ(I)) and wR2 = 0.1773 (all data). The in vitro antimicrobial activities of the compounds were conducted against various Gram-negative, Gram-positive bacteria and fungi. Amongst the tested compounds 7e displayed promising antibacterial activity against Gram-positive bacteria Bacillus flexus compared to antibiotic Amoxicillin [4].
Synthesis of pyrrolopiperidinone acetic acids
Pyrrolopiperidinone acetic acids (PPAs) were identified as highly potent CRTh2 receptor antagonists. In addition, many of these compounds displayed slow-dissociation kinetics from the receptor. Structure–kinetic relationship (SKR) studies allowed optimisation of the kinetics to give potent analogues with long receptor residence half-lives of up to 23 h. Low permeability was a general feature of this series, however oral bioavailability could be achieved through the use of ester prodrugs [5].
References
[1] Horatscheck A, Andrijevic A, Nchinda A T, et al. Identification of 2, 4-disubstituted imidazopyridines as hemozoin formation inhibitors with fast-killing kinetics and in vivo efficacy in the Plasmodium falciparum NSG mouse model[J]. Journal of medicinal chemistry, 2020, 63(21): 13013-13030.
[2] Jose G, Kumara T H S, Sowmya H B V, et al. Synthesis, molecular docking, antimycobacterial and antimicrobial evaluation of new pyrrolo [3, 2-c] pyridine Mannich bases[J]. European Journal of Medicinal Chemistry, 2017, 131: 275-288.
[3] Balfour M N, Franco C H, Moraes C B, et al. Synthesis and trypanocidal activity of a library of 4-substituted 2-(1H-pyrrolo [3, 2-c] pyridin-2-yl) propan-2-ols[J]. European Journal of Medicinal Chemistry, 2017, 128: 202-212.
[4] Jose G, Kumara T H S, Nagendrappa G, et al. Synthesis, crystal structure, molecular docking and antimicrobial evaluation of new pyrrolo [3, 2-c] pyridine derivatives[J]. Journal of Molecular Structure
- Related articles
- Related Qustion
- Exploring the Versatile World of 2-Chloro-3-iodopyridin-4-amine: Insights into Its Structure, Mechanism, and Multifaceted Applications Apr 23, 2024
2-CHLORO-3-IODOPYRIDIN-4-AMINE is a compound that has attracted great attention in the field of chemistry, especially in drug research and development.
The passage introduces the uses, synthesis and hazards of sodium metabisulfite, also mention the reaction with water of it.....
Nov 24,2022Inorganic salts2-CHLORO-3-IODOPYRIDIN-4-AMINE
909036-46-0You may like
2-CHLORO-3-IODOPYRIDIN-4-AMINE manufacturers
- 2-Chloro-3-iodo-4-pyridinamine
-
- $200.00 / 1KG
- 2024-01-02
- CAS:909036-46-0
- Min. Order: 1KG
- Purity: 99%, 99.5% Sublimated
- Supply Ability: g-kg-tons, free sample is available
- 2-CHLORO-3-IODOPYRIDIN-4-AMINE
-

- $1.00 / 1KG
- 2019-08-08
- CAS:909036-46-0
- Min. Order: 1KG
- Purity: 98%
- Supply Ability: 100KG