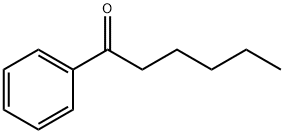
Synthesis and Application of Hexanophenone
Nov 16,2022
Physicochemical property
Hexanophenone has a melting point of 25-26 ℃, so when it melts, it becomes a liquid. It has a boiling point of 265 °C and a density of 0.958 g/mL at 25 °C.
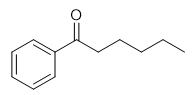
Fig. 1 The structure of Hexanophenone.
Synthetic routes
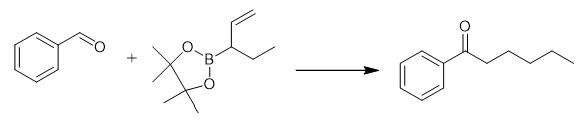
Fig. 2 The synthetic method 1 of Hexanophenone.
Add [Rh (cod) Cl]2 (3.0 mg, 0.006 mmol), Xantphos (6.9 mg, 0.012 mmol) and anhydrous toluene (0.5 mL) to an oven-dried 10 mL resealable pressure tube in a glove box. Stir the resulting solution for 5 min at ambient temperature. Add KHF2 (23.4 mg, 0.3 mmol) and toluene (0.5 mL) and stir the reaction mixture for 20 min. Add benzaldehyde (31.8 mg, 0.3 mmol), 2-4, 4, 5, 5-tetramethyl-2- (pent-1-en-3-yl) -1, 3, 2-dioxaborolane (88.0 mg, 0.45 mmol), MeOH (0.3 mL) and toluene (1.2 mL). Seal the reaction mixture, remove from the glove box and heat at 110°C for 15 h. Cool the resulting suspension to ambient temperature and filter through a silica gel pad. Wash with EtOAc and concentrate in vacuo. Purify the residue by silica gel flash chromatography using eluent: Petroleum ether/EtOAc = 100:1. 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 7.4 Hz, 2H), 7.55 (t, J = 7.4 Hz, 1H), 7.45 (t, J = 7.6 Hz, 2H), 2.96 (t, J = 7.4 Hz, 2H), 1.76 .-. 1.70 (m, 2H), 1.39 .-. 1.34 (m, 4H), 0.94 .-. 0.87 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 200.7, 137.2, 133.0, 128.7, 128.2, 38.7, 31.7, 24.2, 22.7, 14.1 [1].

Fig. 3 The synthetic method 2 of Hexanophenone.
Add Ni(ClO4)2·6H2O (3.6 mg, 0.01 mmol), bipyridine (2.3 mg, 0.015 mmol) to an oven-dried microwave vial (10 mL) equipped with a stir bar (10 x 5 mm) under an argon atmosphere inside a glove box at 25°C. Add 0.1 mL of dry DMA to the mixture via syringe. Stir the catalyst/ligand solution for 1 hour at 25°C inside the glove box. Add Zn powder (13.1 mg, 0.2 mmol, 2 equiv) to the reaction vial. Add cyclopropyl(phenyl)methanone (13.8 μL, 0.10 mmol, 15 μL) and bromoethane (18.7 μL, 0.25 mmol, 2.5 equiv) to the reaction vial. Seal the microwave vial with a cap containing a rubber septum and remove from the glove box. Stir the reaction mixture (~800 rpm) at 40°C in an oil bath for 12 hours. Remove the resulting brown black solution from the oil bath. Cool the mixture to room temperature. Quench the reaction by addition of 5 drops water through the septum via syringe and open the vial to air. Pass the reaction mixture through a plug of silica gel (200-300 mesh). Rinse the silica gel with 5 mL of ethyl acetate to obtain a yellow solution. Remove the solvent and volatile materials by rotary evaporator. Purify the crude residue by flash column chromatography on silica gel (eluted with petroleum ether:EtOAc = 50:1) to obtain 1-phenylhexan-1-one [2].
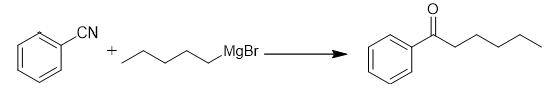
Fig. 4 The synthetic method 3 of Hexanophenone.
Add Grignard reagent (15 mmol) slowly to a solution of alkyne (10 mmol) in dry THF (30 mL) at 0°C. Allow the reaction mixture to stir at room temperature for 16 hours. Quench the reaction by addition of a saturated aqueous NH4Cl solution. Add 1M HCl to the reaction mixture. Stir the reaction mixture for 1 hour. Extract the mixture with Et2O (3 × 100 ml). Combine the combined organic phases. Wash the combined organic phases with brine. Dry the combined organic phases over Na2SO4. Evaporate the solvent under reduced pressure. Purify the crude product by column chromatography on silica gel [3].
Application
As a competitive inhibitor for the enzyme activity
The inhibitory effects of alkyl phenyl ketones on carbonyl reductase activity were examined in pig heart. In this study, carbonyl reductase activity was estimated as the ability to reduce 4-benzoylpyridine to S(-)-alpha-phenyl-4-pyridylmethanol in the cytosolic fraction from pig heart (pig heart cytosol). The order of their inhibitory potencies was hexanophenone. valerophenone. heptanophenone. butyrophenone. propiophenone. The inhibitory potencies of acetophenone and nonanophenone were much lower. A significant relationship was observed between V-max/K-m values for the reduction of alkyl phenyl ketones and their inhibitory potencies for carbonyl reductase activity in pig heart cytosol. Furthermore, hexanophenone was a competitive inhibitor for the enzyme activity. These results indicate that several alkyl phenyl ketones including hexanophenone inhibit carbonyl reductase activity in pig heart cytosol, by acting as substrate inhibitors [4].
References
[1] Zhang K, Huang J, Zhao W. Rh‐Catalyzed Coupling of Aldehydes with Allylboronates Enables Facile Access to Ketones[J]. Chemistry–A European Journal, 2022, 28(15): e202103851.
[2] Cui N, Lin T, Wang Y E, et al. Nickel-Catalyzed Reductive Coupling of γ-Metalated Ketones with Unactivated Alkyl Bromides[J]. Organic Letters, 2022.
[3] Yang J, Massaro L, Krajangsri S, et al. Combined Theoretical and Experimental Studies Unravel Multiple Pathways to Convergent Asymmetric Hydrogenation of Enamides[J]. Journal of the American Chemical Society, 2021, 143(51): 21594-21603.
[4] Imamura Y, Narumi R, Shimada H. Inhibition of carbonyl reductase activity in pig heart by alkyl phenyl ketones[J]. Journal of enzyme inhibition and medicinal chemistry, 2007, 22(1): 105-109.
- Related articles
- Related Qustion
- Hexanophenone: Overview, Appliations in Parmaceutical Cemistry and Safety Dec 11, 2024
Hexanophenone, vital in fragrance and pharmaceutical industries, requires careful handling due to flammability and health hazards, emphasizing the importance of safety protocols.
Hexanophenone
942-92-7You may like
- Hexanophenone
-

- $10.00 / 1ASSAYS
- 2026-02-20
- CAS:942-92-7
- Min. Order: 1ASSAYS
- Purity: 99%
- Supply Ability: 10 ton
- HEXANOPHENONE
-

- $1.00 / 1kg
- 2026-01-30
- CAS:942-92-7
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 10 mt
- Hexanophenone
-

- $0.00 / 1kg
- 2026-01-19
- CAS:942-92-7
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 10T